- Title
-
Notch signaling regulates neural precursor allocation and binary neuronal fate decisions in zebrafish
- Authors
- Shin, J., Poling, J., Park, H.C., and Appel, B.
- Source
- Full text @ Development
|
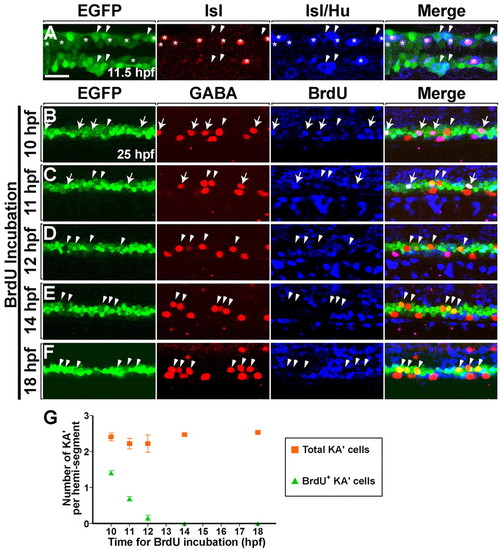
Neural plate olig2:EGFP+ precursors generate PMNs and KA' interneurons at the same time. (A) Dorsal view of the posterior neural plate at 11.5 hpf (five-somite stage) and (B-F) lateral view of 6- to 12-somite regions of spinal cord at 25 hpf in Tg(olig2:egfp) zebrafish embryos. (A) Heterogeneous primary neuronal populations within the EGFP+ domain. Asterisks and arrowheads mark EGFP+ Hu+ Isl+ PMNs and EGFP+ Hu+ Isl- interneurons, respectively. Isl protein is nuclear, whereas Hu is cytoplasmic. (B-F) Embryos were incubated with BrdU at successive timepoints (as shown to the left of each panel) and labeled with anti-GABA (red) and anti-BrdU (blue) antibodies. Arrows and arrowheads mark BrdU+ and BrdU- KA' interneurons, respectively. (B) Four KA' interneurons were formed from EGFP+ precursors that underwent S phase at 10 hfp (arrows). Arrowhead marks BrdU- KA' interneuron, indicating that the postmitotic cell was formed before or after 10 hpf. (C) Two BrdU+ KA' interneurons (arrows) and two BrdU- KA' interneurons (arrowheads) were detected in the embryos that were incubated with BrdU at 11 hpf. (D,E,F) Embryos incubated with BrdU at 12, 14 and 18 hpf. No BrdU+ KA' interneurons were evident. (G) Average of all KA' cells (squares) versus BrdU+ KA' cells (triangles) (n=4 animals each). S phase for the last KA' interneurons produced occurs between 10 and 12 hpf. Error bars represent s.e.m. Scale bar: 25 µm. |
|
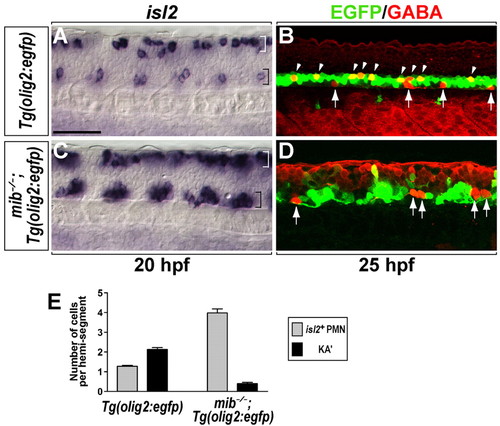
Loss of Notch signaling causes formation of excess PMNs and a deficit of KA' interneurons. (A-D) Lateral views of the spinal cord at the 6- to 12-somite region of zebrafish embryos, with anterior to left and dorsal at the top. (A) A subset of PMNs (CaP and VaP) and Rohon-Beard neurons of Tg(olig2:egfp) embryos expressed isl2 in the ventral spinal cord (black bracket) and dorsal spinal cord (white bracket) at 20 hpf, respectively. (B) In Tg(olig2:egfp) embryos, KA' interneurons (arrowheads) were detected by anti-GABA antibody (red) within EGFP+ cells at 25 hpf. EGFP- GABA+ cells (arrows) in ventral spinal cord are KA'' interneurons (Park et al., 2004). (C) In mib-/-;Tg(olig2:egfp) embryos, isl2+ PMNs formed as clusters in each hemisegment in the ventral spinal cord (black bracket). (D) mib-/-;Tg(olig2:egfp) embryos did not produce EGFP+ GABA+ KA' interneurons, although EGFP- GABA+ KA'' interneurons were still present (arrows). (E) Mean of the number of PMNs (gray bar) and KA' interneurons (black bar) per hemisegment in Tg(olig2:egfp) and mib-/-;Tg(olig2:egfp) embryos (n=7). Error bars represent s.e.m. Scale bar: 50 µm. PHENOTYPE:
|
|
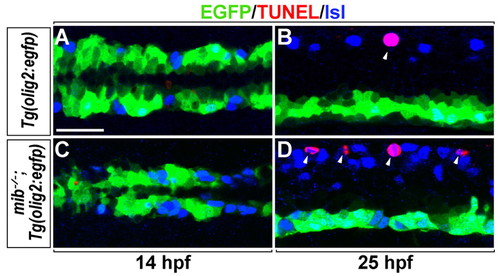
Deficit of KA' interneurons is independent of apoptosis in mib-/- zebrafish embryos. Dorsal views of the posterior neural plate at 14 hpf (A,C) and lateral views of spinal cord at 25 hpf (B,D) in Tg(olig2:egfp) (A,B) and mib-/-;Tg(olig2:egfp) embryos (C,D). Green, blue and red channels mark EGFP+ cells, Isl+ neurons and TUNEL+ cells, respectively. Neither control nor mib-/- embryos had EGFP+ TUNEL+ cells at 14 hpf or 25 hpf (A-D). TUNEL+ Rohon-Beard neurons (arrowheads) were detected in control and mib-/- embryos (B,D) at 25 hpf. Scale bar: 25 µm. |
|
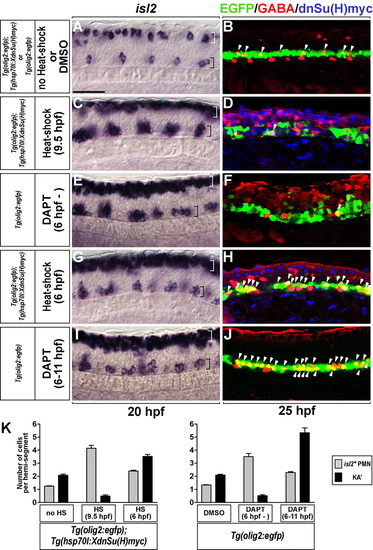
Transient inactivation of Notch signaling produces excess PMNs and KA' interneurons. (A-J) Lateral views of the spinal cord of zebrafish embryos at the 6- to 12-somite region, with anterior to the left and dorsal at the top. All embryos carried the Tg(olig2:egfp) transgene. (C,D,G,H) Tg(olig2:egfp);Tg(hsp70l:XdnSu(H)myc) double-transgenic embryos. (A,C,E,G,I) isl2 expression at 20 hpf. White and black brackets mark dorsal and ventral spinal cord, respectively. (B,D,F,H,J) KA' cells (arrowheads) were detected by anti-GABA antibody (red) within EGFP+ cells (green) at 25 hpf. (A,B) Control embryos. (C,D) Induction of XdnSu(H)Myc by heat shock at 9.5 hpf resulted in excess PMNs with reduction of KA' interneurons (arrowhead). XdnSu(H)Myc protein was detected by anti-Myc antibody (blue). (E,F) Embryos incubated continuously with DAPT had excess PMNs and a deficit of KA' interneurons (arrowhead). (G,H) Double-transgenic embryos heat shocked at 6 hpf formed excess PMNs (black bracket) and KA' interneurons (arrowheads). (I,J) Embryos incubated with DAPT from 6 to 11 hpf had excess PMNs (black bracket) and KA' cells (arrowheads). (K) Mean of the number of PMNs (gray bar) and KA' interneurons (black bar) per hemisegment (n=7) in A-J. Error bars represent s.e.m. Scale bar: 50 µm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
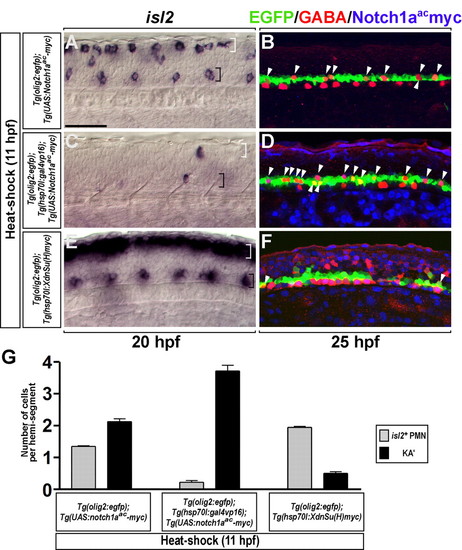
Conditional activation of Notch signaling produces excess KA' interneurons at the expense of PMNs. (A-F) Lateral views of the spinal cord of zebrafish embryos at the 6- to 12-somite region, with anterior to the left and dorsal at the top. (A,B) Control Tg(UAS:Notch1aac-myc);Tg(olig2:egfp), (C,D) Tg(hsp70l:gal4vp16);Tg(UAS:Notch1aac-myc);Tg(olig2:egfp) and (E,F) Tg(hsp70l:XdnSu(H)myc);Tg(olig2:egfp) embryos were heat shocked at 11 hpf. (A,B) Control embryos showed normal numbers of isl2+ PMNs (black bracket), dorsal Rohon-Beard sensory neurons (white bracket) and GABAergic KA' cells (arrowheads). (C) isl2+ PMNs (black bracket) and Rohon-Beard neurons (white bracket) were almost absent from heat-shocked triple-transgenic embryos. (D) Heat-shocked triple-transgenic embryos had excess KA' interneurons (arrowheads). (E,F) Heat-shocked Tg(hsp70l:XdnSu(H)myc);Tg(olig2:egfp) embryos had excess PMNs (black bracket), excess Rohon-Beard neurons (white bracket) and a deficit of KA' interneurons (arrowheads). (G) Mean of the number of PMNs (gray bar) and KA' interneurons (black bar) per hemisegment (n=7) in A-F. Error bars represent s.e.m. Scale bar: 50 µm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
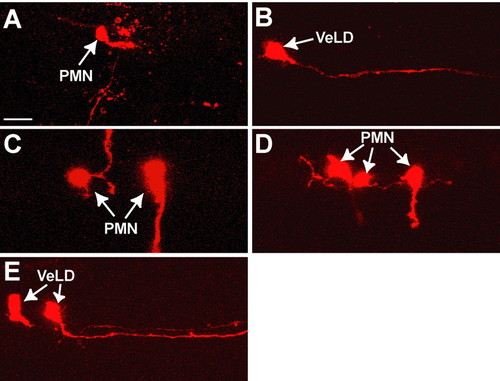
Individual olig2:EGFP+ neural plate cells produce single types of neurons in mib-/- embryos. (A-E) Lateral views of the spinal cord of 24- to 30-hpf mib-/-;Tg(olig2:egfp) zebrafish embryos, with anterior to left and dorsal at the top. In each case, a single EGFP+ cell was labeled with vital dye (red) at 10 hpf. (A,B) Labeled cells that did not divide but differentiated into a PMN and VeLD. (C,D) Examples of individual EGFP+ clones that produced only PMNs, as determined by axons that projected out of the spinal cord to muscle. (E) Example of an EGFP+ clone that produced two VeLD interneurons. Scale bar: 20 µm. |
|
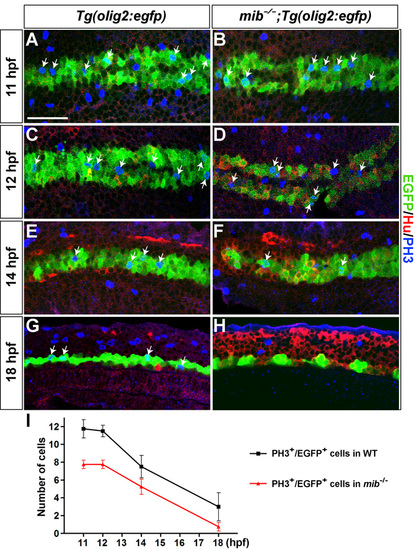
Proliferation of EGFP+ cells in mib-/-;Tg(olig2:egfp) embryos. Dorsal views of the posterior neural plate at 11 (A,B), 12 (C,D) and 14 hpf (E,F) and lateral views of spinal cord at 18 hpf (G,H) in Tg(olig2:egfp) (A,C,E,G) and mib-/-;Tg(olig2:egfp) embryos (B,D,F,H). Green, blue and red channels mark EGFP+ cells, PH3+ mitotic cells and Hu+ postmitotic neurons, respectively. Arrows indicate PH3+ EGFP+ cells that underwent M phase of cell division. (I) Average of PH3+ EGFP+ cells in control (squares) and mib-/- embryos (triangles) (n=4 animals each). Error bars represent s.e.m. Scale bar: 50 μm |
|
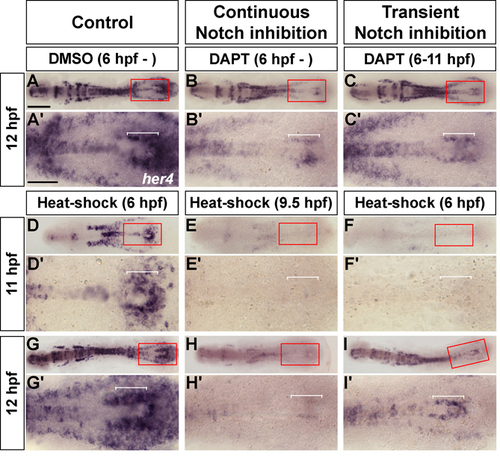
Recovery of Notch signaling after transient inhibition. Dorsal views of flat-mounted zebrafish embryos, labeled by her4. A′-I′ are magnified views of boxed areas in A-I. (A,A′) Control embryo showing normal expression of her4 at 12 hpf. Bracket marks her4-expressing neuroectoderm cells. (B,B′) Continuous DAPT incubation caused significant reduction in her4 expression in posterior neuroectoderm (bracket). (C,C′) Embryos incubated with DAPT from 6 to 11 hpf re-expressed her4 (bracket) at 12 hpf. (D-F′) 11 hpf embryos. (D,D′) Control embryo. (E,E′) After heat shock at 9.5 hpf, Tg(hsp70l:XdnSu(H)myc) embryos did not express her4. (F,F′) After heat shock at 6 hpf, transgenic embryos did not express her4. (G-I′) 12 hpf embryos. (G,G′) Control embryo. (H,H′) After heat shock at 9.5 hpf, transgenic embryos did not express her4. (I,I′) After heat shock at 6 hpf, her4 expression was evident in posterior neuroectoderm in transgenic embryos. Scale bar: in A, 200 μm for A-I; in A′, 100 μm for A′-I′. PHENOTYPE:
|