- Title
-
Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes
- Authors
- Zhu, Y., Rice, C.D., Pang, Y., Pace, M., and Thomas, P.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
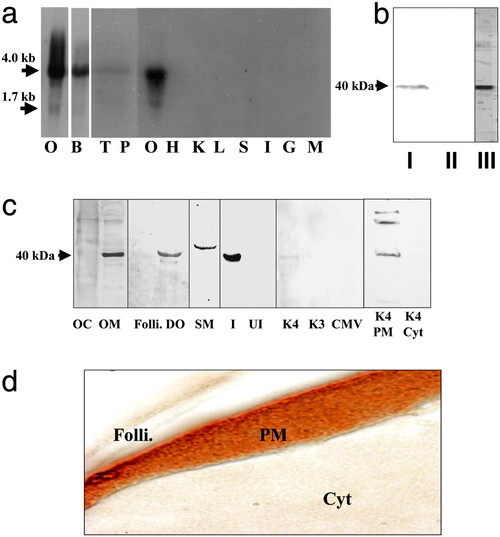
Tissue distribution and cellular localization of putative mPR. (a) Northern blot analysis showing mRNA expression in ovarian (O, gel loading: 1 μg) and other tissues (gel loading: 5 μg); B, brain; T, testis; P, pituitary; H, heart; K, kidney; L, liver; S, stomach; I, intestine; G, gill; M, muscle. (b) Western blot analysis of solubilized ovarian membrane proteins using monoclonal antibody PR10-1. I, plasma membrane; II, cytosol; III, partially purified membrane fraction used to generate monoclonal antibodies. (c) Cellular localization of receptor by Western blot analysis using stmPRαpAb1 antibody (gel loading: 10 μg). OC, oocyte cytosol; OM, oocyte membrane; Folli., follicle cell membrane; DO, denuded oocyte plasma membrane; SM, sperm membrane; I, recombinant protein induced by IPTG in E. coli; UI, noninduced E. coli protein; K4, membrane proteins from mPR-transfected MDA-MB-231 cells; K3, control cells transfected with vector containing reverse insert; CMV, control cells transfected with empty carrier vector. The following lanes were probed with PR10-1 antibody: K4 PM, plasma membrane from mPR transfected MDA-MB-231 cells; K4 Cyt, cytosol from mPR transfected cells. (d) Immunocytochemical localization of mPR receptor protein in a mature seatrout follicle containing a stage IV oocyte using stmPRαpAb1 antibody. Folli., follicle cells; PM, oocyte plasma membrane; Cyt, oocyte cytoplasm. (Magnification: 1 cm ≈ 20 μm.) |
|
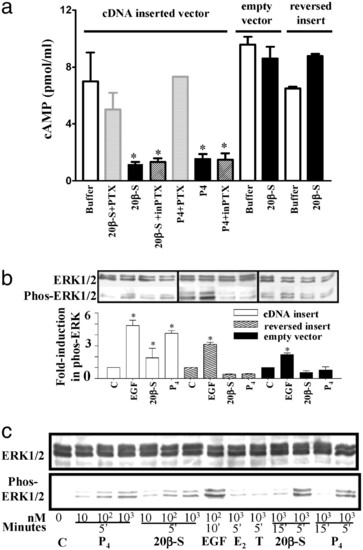
Inhibition of cAMP production (a) and activation of MAP kinase signaling pathway (b and c) in response to progestin hormones in MDA-MB-231 cells stably transfected with putative mPR cDNA (means ± SEM, n = 4; *, P < 0.05). (a) Cells were preexposed to activated pertussis toxin (PTX) or inactive pertussis toxin (inPTX) for 30 min at 37°C before incubation with 20β-S or progesterone for 5 min. (b) MAP kinase activity (shown as increase in phospho-Erk1/2) in cells transfected with vector containing mPR insert (white bar), controls containing reversed insert (shaded bar) or vector alone (black bar) after a 5-min stimulation with 1 μM 20β-S or progesterone (P4). C, untreated control; EGF, human epidermal growth factor (1 μM, positive control). (c) Time- and dose-dependent activation of MAP kinase (gel loading: 20 μg per lane) in transfected cells treated with 20β-S, P4, estradiol-17β (E2), or testosterone (T). |
|
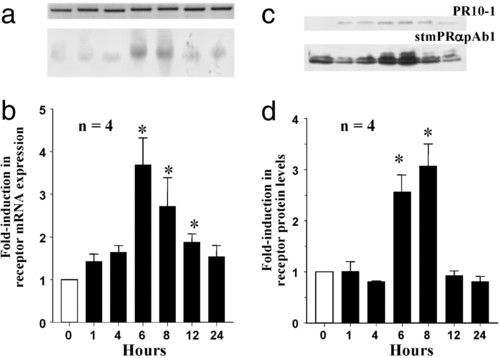
Time course of changes in putative mPR mRNA (a and b) and protein levels (c and d) in ovarian fragments after in vitro treatment with 300 nM 207beta;-S, relative to control (0 h) values (mean ± SEM, n = 4; *, P < 0.05). (a) Representative Northern blot (gel loading: 1 μg). (Lower) Putative mPR mRNA. (Upper) Corresponding β-actin mRNA. (b) Relative levels of mPR mRNA. (c) Representative Western blot analyses using PR10-1 and stmPRαAb1 antibodies (gel loading: 10 μg). (d) Relative levels of mPR protein. |
|
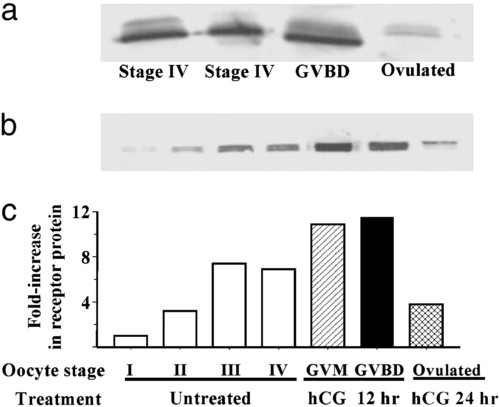
Changes in putative mPR protein levels in oocytes at various stages of development and final maturation detected by Western blot analysis with stmPRαAb1 antibody (gel loading: membrane proteins from five oocytes per lane). (a) In wild fish captured on their spawning grounds. (b) After incubation in vitro in the presence or absence of hCG. I, vitellogenic oocyte, 200-300 μm (diameter); II, 300-375 μm; III, 375-440 μm; IV, full-grown oocyte, 430-500 μm; GVM, germinal vesicle migration stage of final maturation; GVBD, germinal vesicle breakdown stage of final maturation. (c) mPR protein levels expressed relative to stage I oocyte values. |
|
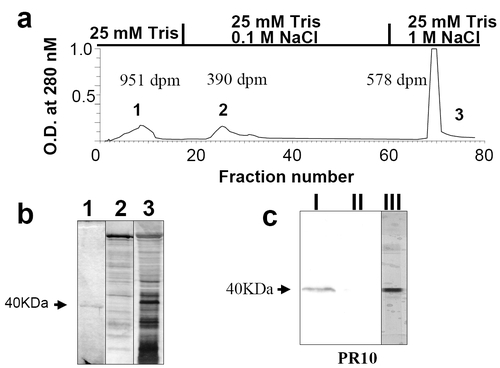
Partial purification of ovarian progestin receptor and monoclonal antibody screening. (a) Elution profile of solubilized seatrout ovarian membrane extracts on a DEAE column showing peaks of UV absorbance and progestin (17,20β,21-trihydroxy-4-pregnen-3-one) binding activity. Each peak was pooled and concentrated with a 10-kDa molecular mass cutoff filter. Specific progestin binding of each pooled fraction is indicated near each peak. (b) Silver stain of SDS/PAGE gel of the three pooled fractions. Numbers refer to the pooled fractions obtained from DEAE chromatography. (c) Western blot of solubilized ovarian membrane proteins using monoclonal antibody PR10-1. I, solubilized seatrout ovarian plasma membrane proteins; II, seatrout ovarian cytosolic proteins; III, partially purified membrane proteins in fraction 1. |