- Title
-
Mezzo, a paired-like homeobox protein is an immediate target of Nodal signalling and regulates endoderm specification in zebrafish
- Authors
- Poulain, M. and Lepage, T.
- Source
- Full text @ Development
|
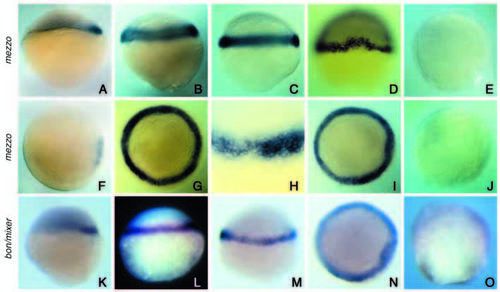
Analysis of mezzo expression in wildtype embryos and comparison with bon/mixer. (A,F,K) Sphere stage (4 hpf), (B,G,L) 30% epiboly (4.7 hpf), (C,H,M) 50% epiboly (5.3 hpf), (D,I,N) shield stage (6 hours), (E,J,O) 70% epiboly (8 hpf). (A-E,K-M,O) Side views. (G,I,J,N) Views from the animal pole. (F,D) Dorsal views. mezzo (A,F) and bon/mixer (K) start to be expressed in a small group of cells on the dorsal side at the sphere stage. At 30% epiboly, expression of mezzo (B,G) and mixer (L) has extended over the whole marginal region. A close examination of mezzo expression at 50% epiboly (H) shows that mezzo transcripts are present in about six rows of cells at the margin of the blastoderm. No expression is detected in the YSL (C,H,M). At the shield stage, mezzo and mixer continue to be expressed throughout the marginal region and in the dorsal shield (D,I,N). At 70% epiboly, mezzo and mixer have been turned off and transcripts are no longer detected in the embryo (E,J,O). EXPRESSION / LABELING:
|
|
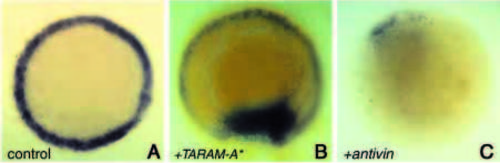
Expression of mezzo is regulated by a TGFβ/TARAM-A signalling pathway. Animal pole views of embryos at 50% epiboly showing mezzo in situ hybridisation (purple). (A) Control uninjected embryo. (B) Embryo injected at the eight-cell stage into one blastomere with 40 pg of TARAM-A* and with 100 pg of mRNA encoding a nuclear β-Gal (blue colour) as a lineage tracer. TARAMA* induces confluent patches of mezzo-expressing cells. (C) Expression of mezzo transcripts was barely detectable in embryos injected with 50 pg of antivin mRNA. EXPRESSION / LABELING:
|
|
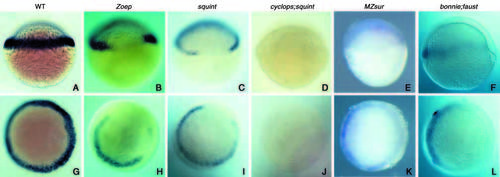
Analyses of mezzo expression in various mutants of the Nodal signalling pathway. All the embryos were fixed at the shield stage except those in B,H (40% epiboly). (A-F) Side views, (G-L) animal pole views. (A,G) Control wild-type embryos. Expression of mezzo was lost on the dorsal side in Zoep (B,H) and squint (C,I) mutant embryos. Expression of mezzo was abolished in cyc;sqt double mutants (D,J) and variably affected in MZsur embryos. The variable expression of mezzo in MZsur correlated with the expressivity of the schmalspur phenotype. The embryos shown in E,K derived from a cross producing severely affected embryos. Expression of mezzo is greatly diminished in two thirds of the marginal region in bon;faust double mutants (F,L). EXPRESSION / LABELING:
|
|
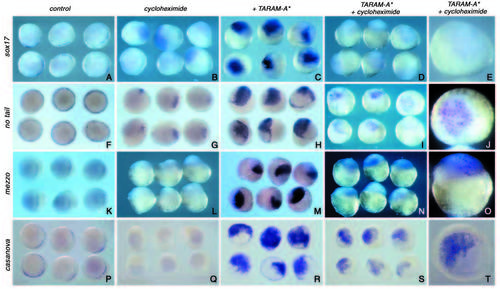
mezzo, casanova, bon/mixer and ntl are immediate early targets of Nodal signalling, while sox17 requires post MBT protein synthesis. Embryos at the one- to four-cell stage were microinjected with 50 pg of TARAM-A* mRNA, treated continuously with 50 mg/ml of cycloheximide, starting at the 64- to 128-cell stage and analysed at dome-30% epiboly stage for the expression of sox17 (A-E), ntl (F-J), mezzo (K-O) and casanova (P-T). (A,F,K,P) Untreated and uninjected control embryos. (B,G,L,Q) Uninjected control embryos treated with cycloheximide. (C,H,M,R) Control untreated embryos injected with TARAM-A*. (D,E,I,J,N,O,S,T), Embryos injected with TARAM-A* and treated with cycloheximide. Although treatment with cycloheximide prevents induction of sox17, some ectopic mezzo, ntl, bon/mixer and cas expression is clearly visible in absence of protein synthesis. EXPRESSION / LABELING:
|
|
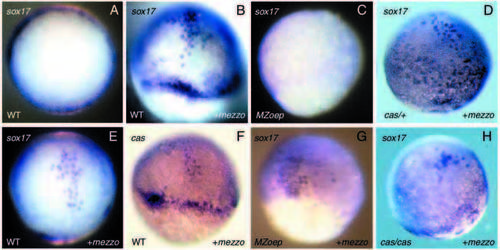
Induction of endodermal marker genes induced by ectopic mezzo in wild-type embryos, MZoep mutants and casanova mutants. (A,E) animal pole views. (B-D,F-H) Side views. Embryos were injected at the 2- to 16-cell stage with 40 pg of GFP (A) or 40 pg of mezzo mRNA and examined at the shield (A,B,E,F) or at 80% epiboly (C,D,G,H) for the expression of sox17 (B,C,D,E,G,H) or casanova (F). Ectopic mezzo induces robust expression of sox17 (B,D,E) or casanova (F). These cells were typically large and scattered like the endogenous endoderm precursors; however, most of those cells remained in the epiblast. Overexpression of mezzo rescues sox17-expressing cells in MZoep (G) and weakly induces ectopic expression of sox17 in casanova (H). |
|
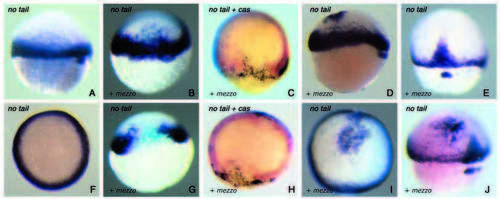
Effects of mezzo mRNA injection on ntl expression. Overexpression of mezzo induces ectopic expression of ntl in the animal hemisphere and repress endogenous ntl at the margin. (A,B,C,D,E,G,J) Side views and (F,H,I) animal pole views of embryos at the shield stage (A-D,F-I) or 60% epiboly (E,J). (A,E,F) Control uninjected embryos. (B,G) Embryos injected at the eight-cell stage with 40 pg of mezzo plus β-Gal RNA. (B) Expansion of the territory expressing ntl caused by injection of mezzo mRNA in the non marginal region. A close examination of these embryos showed the presence of the lineage label outside the margin. (G) Repression ntl expression caused by injection of mezzo in the marginal region. Note the presence of the lineage label in the sector of cells that do not express ntl. (C,H) Two-colour in situ hybridisation showing induction of casanova (brown) and repression of ntl (red) after overexpression of mezzo in the marginal region. (D,I,J) Injection of mezzo mRNA at the same concentration as above into one central blastomere of embryos at the 16-cell stage induced patches of ntl expression in the animal hemisphere. |
|
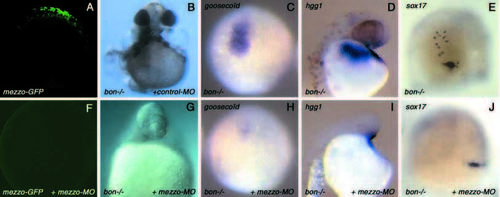
Injection of an antisense morpholino oligonucleotide directed against mezzo in bon mutants. (A) Control embryo injected with 100 pg of an mRNA encoding a fusion protein between the 5′ leader sequence of mezzo and the sequence coding for the first 43 amino acids fused to the coding sequence of the green fluorescent protein to make mezzo-GFP mRNA. (F) Embryo injected with 100 pg of mezzo-GFP mRNA and with 12 ng the antisense morpholino directed towards the 5′ end of mezzo (mezzo-MO). All the embryos injected with mezzo-GFP mRNA were brightly fluorescent when examined at the shield stage (A). In embryos co-injected with mezzo-GFP and 12 ng of mezzo-MO, the fluorescence was abolished (F). Note that the camera gain in this image was much higher than for the image in A. Using the same settings as A, the image would be black. (B) bon mutants injected with an unrelated morpholino directed against the sea urchin hatching enzyme mRNA used as a negative control. (G) bon mutant embryo injected with 12 ng of antisense morpholino targeted against mezzo. (C,H) Expression of the prechordal plate marker goosecoid in control uninjected (C) and in morpholino injected (H) bon mutant embryos at 80% epiboly. (D,I) Expression of the hatching gland marker hgg1 in control uninjected (D) and in morpholino injected (I) bon mutants. (E,J) Residual sox17 expression in homozygous bon mutants at 80% epiboly (E), and absence of sox17-expressing cells in bon mutants injected with the morpholino (J). To unambiguously identify homozygous bon mutants, their genotype was determined by PCR (Kikuchi et al., 2001). EXPRESSION / LABELING:
PHENOTYPE:
|
|
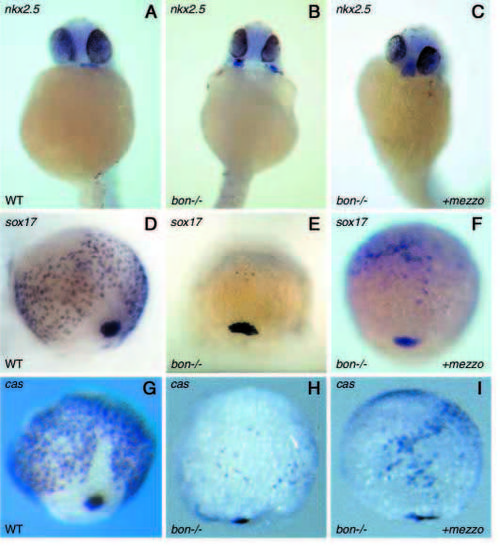
Overexpression of mezzo partially rescues heart morphogenesis in bon mutants and increases the number of sox17- and cas-expressing cells. (A-C) Rescue of heart morphogenesis after injection of mezzo mRNA into bon mutant embryos monitored by expression of nkx2.5 at 30 hours. The failure of heart primordia to migrate and fuse in the midline in bon mutants (B) was partially restored by overexpression of mezzo (C). (D-I) Dorsal views of embryos at 80% epiboly, showing expression of endodermal markers in wild-type embryos (D,G), bon mutant embryos (E,H) and bon mutant embryos injected with mezzo mRNA (F,I). Embryos were injected with 20-40 pg of mezzo mRNA a the one- to four-cell stage and examined for expression of sox17 (F) or casanova (I). The number of sox17- or casanova-positive cells is significantly increased in homozygous bon mutants injected with mezzo mRNA (F,I) compared with control uninjected mutant embryos (E,H). bon mutant embryos were genotyped by PCR. EXPRESSION / LABELING:
PHENOTYPE:
|