- Title
-
Specification and morphogenesis of the zebrafish larval head skeleton
- Authors
- Kimmel, C.B., Miller, C.T., and Moens, C.B.
- Source
- Full text @ Dev. Biol.
|
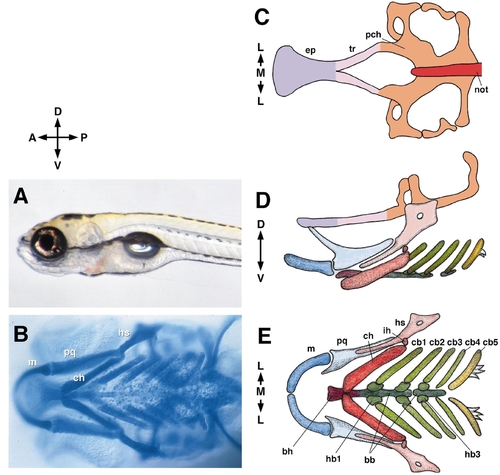
The young larval zebrafish (A, 5 days postfertilization, left side view) and the layout of its cartilaginous head skeleton. For a further description see Schilling and Kimmel (1997) and Cubbage and Mabee (1996). Anterior is to left in this and other figures in the paper except where indicated. B shows a ventral view of an Alcian blue-labeled, whole-mounted preparation. Cartilages are indicated in the first or mandibular arch (pq, m) and in the second or hyoid arch (hs, ch). C–E show drawings of such preparations. (C) The neurocranial (or basicranial) cartilages and notochord from the dorsal aspect. The eyes fit into the shallow grooves along the sides of the ethmoid plate and trabeculae. The otic vesicles fit into the prominent cavities to either side of the notochord and parachordal cartilages . The brain’s posterior pituitary fits into the prominent midline cavity ahead of the notochord, the hypophysial fenestra. (D). The neurocranium (diagrammatically elevated dorsalward for the sake of clarity) and the pharyngeal skeleton in side view. (E). The pharyngeal skeleton in ventral view. Abbreviations used: A, anterior; bb, basibranchial; bh, basihyal; cb, ceratobranchial; ch, ceratohyal; D, dorsal; ep, ethmoid plate; hb, hypobranchial; hs, hyosymplectic; ih, interhyal; L, lateral; M, medial; m, Meckel’s; not, notochord; P, posterior; pch, parachordal; pq, palatoquadrate; tr, trabecula; V, ventral. |
|
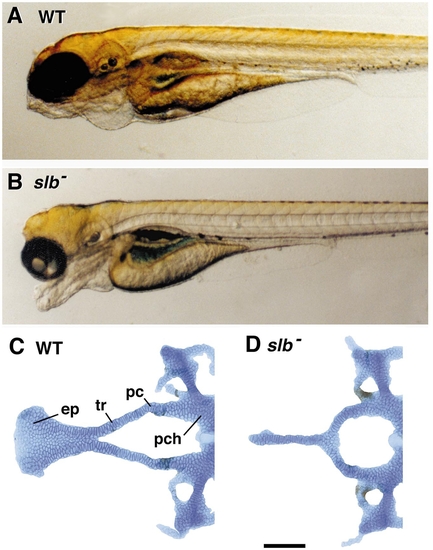
The “bulldog-headed zebrafish,” comparing the wild-type (WT, A) and slb mutant (B) embryos at 2 days of development (unpublished photographs, courtesy of Dr. Corinne Houart). (C, D) Basicranial cartilages labeled with Alcian blue, dissected, and laid out as a flat mount, viewed from the dorsal aspect (unpublished, B. Ullmann and C.B.K.); pc, polar cartilage region; a distinctive region of joining between the parachordals and trabeculae. The polar cartilages form as separate elements in some organisms (e.g., see De Beer, 1937; Goodrich, 1930), but probably not in zebrafish. The abbreviations are as in Fig. 1. |
|
Cartilage phenotypes and dlx2 expression in WT and lzr mutants, (from Pöpperl et al., 2000). (A, B) Alcian blue-stained, flat-mounted cartilages of the first and second pharyngeal segments prepared from day 4 embryos. Left side views, dorsal to the top and anterior to the left. In the WT (A) the elements are separated by joints. The abbreviations are as in Fig. 1. In the mutant (B) the dorsal elements as well as the ventral elements in the two segments are fused to one another. DV fusions within each segment occur as well; the DV joints are missing, as is the interhyal cartilage (ih) in the second segment. (C, D) Dorsal views (anterior to the left) of 20-h embryos labeled by RNA in situ hybridization for expression of dlx2. The three separate bilateral patches of expression in the WT (C) correspond to the three streams of postmigratory neural crest that have populated the pharyngeal arch primordia. The streams are fused together in the lzr mutant. |
|
Cartilage phenotypes and hox gene expression in WT and val mutants. (A–E) Ventral views, anterior to the top, of flat-mounted cartilages in 7-day-old larvae (from Moens et al., 1998). (A) The WT pharyngeal segments 2–7. The hyoid segment (second pharyngeal segment) uniquely contains a small interhyal (ih) cartilage. At this stage, there is no separate hypohyal cartilage, the serial homolog of the hypobranchials. The ceratohyal (ch) is distinctively larger in size than its segmental homologs, the ceratobranchials (cb1–5). Ceratobranchials are tapering elements, as shown in B for cb1 (third pharyngeal segment) in another WT preparation at higher magnification. (C–E) The cartilages in the same (third) segment in three individual val mutants. The principal element, normally a ceratobranchial is often truncated and thickened, now more like a ceratohyal (C). Separate hypobranchials may be missing (arrow in D), and small interhyal-like elements are sometimes present (arrowheads in D and E). (F–I) hox gene expression in WT (F, H) and val (G, I) mutants, dorsal views with anterior to the left (from Prince et al., 1998). (F, G) hoxb2 at 19 h (20-somite stage). (H, I) hoxb3 expression at 14 h (10-somite stage). The more anterior gene hoxb2 (F, G) is ectopically expressed in neural crest that will populate the third pharyngeal segment (arrowhead in G). The more posterior gene hoxb3 (H, I) normally has a distinctive domain of strong expression in rhombomeres 5 and 6, and this domain is missing in the mutant (arrowheads). |
|
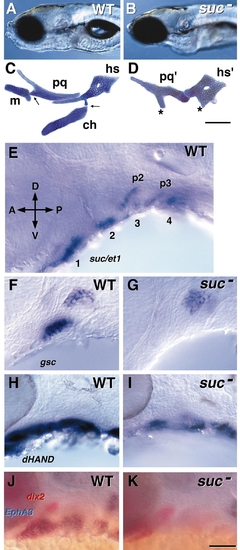
Phenotype and gene expression in WT and suc mutants (from Miller et al., 2000). WT are to the left and suc mutants to the right. (A, B) The craniofacial appearance in left side view of WT and a suc mutant at day 4. (C, D) The cartilage phenotype at day 4. Alcian blue-labeled, flat-mounted elements of the mandibular and hyoid arches (pharyngeal segments 1 and 2). The abbreviations are as in Fig. 1. The asterisks in D show regions of ventral cartilage that may correspond to m and ch in the first and second segments, respectively. Scale bar, 100 μm. (E) Whole mount RNA in situ preparation (36 h, left side view) showing segmental expression of suc/et-1 in the ventralpharyngeal arches. The first four segments are indicated (1– 4), and labeling is also present in endodermal pouches 2 and 3 (p2, p3). (F–K) Targets of suc signaling, as revealed by comparing the RNA expression patterns in WT and suc mutants. Left side views of whole mounted embryos. (F, G) goosecoid (gsc), 30 h. (H, I) dHAND, 28 h. (J, K) dlx2 (in red) and EphA3 (in blue), 32 h. Scale bar (K), 50 μm. |
|
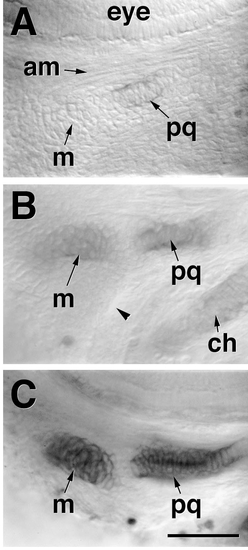
Chondrification of the dorsal (pq) and ventral (m) cartilages within a single precartilage condensation in the mandibular segment. Left side views of Alcian blue-labeled whole-mounted embryos, photographed with Nomarski optics (from Schilling and Kimmel, 1997). (A) 53 h. The eye is at the top of the field and the rudiment of the adductor mandibular muscle (am, mediating movement between pq and m) is visible. Other abbreviations are as in Fig. 1. The dorsal cartilage, the palatoquadrate (pq), has begun to chondrify (become Alcian-positive) within the same mandibular condensation that will also form Meckel’s cartilage (m). An unlabeled bridge of condensed mesenchyme, present just beneath the muscle rudiment, connects these two labeled regions and shows there is but a single condensation. See also Fig. 10, for the appearance of the condendensation in a live embryo. This bridging region will eventually develop as the joint between pq and m. (B) Another embryo at about the same stage in which m is also lightly labeled, and the joint-forming bridge remains unlabled. The arrowhead indicates the hyomandibular pouch, separating the mandibular and hyoid arches. (C) 60 h. Both cartilages, but not the joint region between them, are strongly labeled. Scale bar, 50 μm. |
|
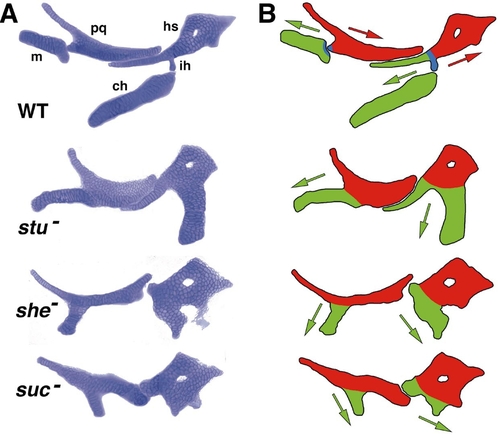
A phenotypic series of anterior arch mutants, arranged in approximate order of severity (unpublished examples, C.T.M; see also Kimmel et al., 1998). (A) Alcian-stained flat mounts of mandibular and hyoid cartilages. Abbreviations and orientation are as in Fig. 1D. The three mutations are at three separate loci; sucker (suc) encodes Endothelin-1; sturgeon (stu) and schmerle (she) have not been identified molecularly. The mutants are arranged by severity of the phenotypes, as judged by the amount of cartilage present in the mutants; stu- is the least severe and suc- the most severe. (B) Drawings illustrating our interpretation of the mutant phenotypes: Red, dorsal cartilages in the two segments; green, ventral cartilages; blue, the DV joint region, missing in mutants. We propose there is a change in polarity of the ventral cartilages in the mutants (see text). By this hypothesis, in WT the dorsal and ventral cartilages have opposite polarities. The polarities of the ventral cartilages (green arrows), which we suppose indicates their direction of outgrowth from the precartilage condensations, become progressively more like that of the dorsal elements (red arrows) with increasing severity of the phenotypes. |
|
Morphogenesis (chondrogenesis) in the anterior pharyngeal wall, as revealed by BODIPY ceramide labeling in live WT preparations (left side view). (A) 52 h, (B) 58 h (unpublished confocal micrographs, M. Jones and C.B.K.). Negative images are shown, allowing the various regions of developing cartilages to be pseudocolored for clarity of presentation. The mature cartilages are shown in the same orientation in Fig. 1D. Here the two distinctive regions of the dorsal hyoid cartilage (the hyosymplectic, Fig. 1) are indicated separately, the strut-like symplectic region (sy) and the plate-like hyomandibular region (hm). The more dorsal part (hyomandibular region; for more detail see Kimmel et al., 1998) is left uncolored. Both of these cartilage-forming regions, as well as the ventral cartilage in this segment, the ceratohyal (ch), are developing out of a common primordium, the hyoid condensation. In contrast, the palatoquadrate (pq) and Meckel’s cartilage (only partly included here) can be seen to be developing out of another shared primordium, the mandibular condensation, as described previously from analysis of fixed material (Schilling and Kimmel, 1997; see also Fig. 8). |
|
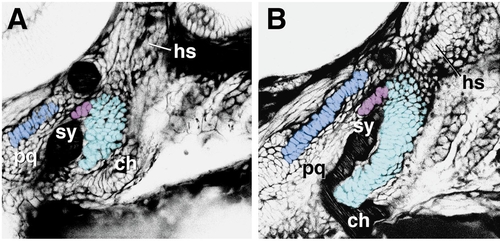
Abnormally curving chondrocyte stacks in the ventral region of the hyoid cartilage of a suc mutant embryo, compared to the pattern in aWT (to the left). Arrows indicate proposed polarities (see text), as in Fig. 9. We propose that the abnormal polarities of the ventral cartilages when Suc/Et-1 signaling is defective is underlain by abnormal orientation of stacking of the chondrocytes (modified from Kimmel et al., 1998). hm and sy, hyomandibular and symplectic regions of the hyosymplectic cartilage. Other abbreviations are as in Fig. 1. Scale bar, 100 μm. |
Reprinted from Developmental Biology, 233(2), Kimmel, C.B., Miller, C.T., and Moens, C.B., Specification and morphogenesis of the zebrafish larval head skeleton, 239-257, Copyright (2001) with permission from Elsevier. Full text @ Dev. Biol.