- Title
-
Follistatin and Noggin are excluded from the zebrafish organizer
- Authors
- Bauer, H., Meier, A., Hild, M., Stachel, S., Economides, A., Hazelett, D., Harland, R.M., and Hammerschmidt, M.
- Source
- Full text @ Dev. Biol.
|
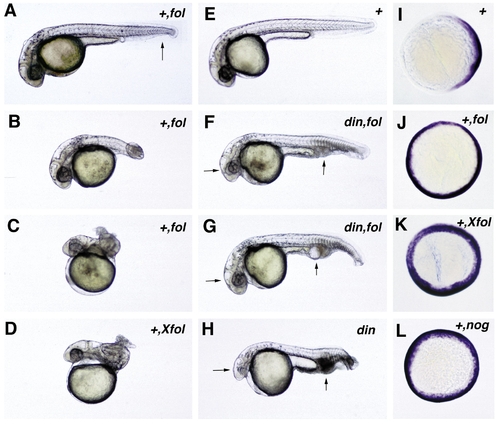
Functional analysis of zebrafish follistatin and noggin. Both molecules can dorsalize zebrafish embryos. (A–D) Overexpression of zebrafish and Xenopus follistatin in wild-type embryos leads to a dose-dependent dorsalization. Weakly dorsalized embryos (C1) display a partial loss of the ventral tail fin, as indicated in (A) by an arrow. Moderate dorsalization (C3) is characterized by a wound-up tail, the “piggy tail” (Mullins et al., 1996) phenotype (B), while in strongly dorsalized embryos (C4/C5), the entire embryonic axis is wound up in a snailshell-like fashion (C, D). (E–H) chordino mutant embryos can be rescued by overexpression of zebrafish follistatin (F, G). Note the normalization of eye sizes and blood islands (indicated by arrows in F–H). All embryos in (A–H) are shown at approximately 36 hpf. (I–L) Expression of fkd3; 70% epiboly, animal view, dorsal right; in the wild-type embryo. (I) fkd3 expression is confined to the dorsal side, while it is expanded into ventralmost regions in embryos injected with zebrafish follistatin (J), Xenopus follistatin (K), or zebrafish noggin (L). Abbreviations: +, wildtype; hpf, hours after fertilization; din,chordino mutant dintt250. The second abbreviation in the upper right corner indicates the injected mRNA: fol, zebrafish follistatin; Xfol, Xenopus follistatin; nog, zebrafish noggin. |
|
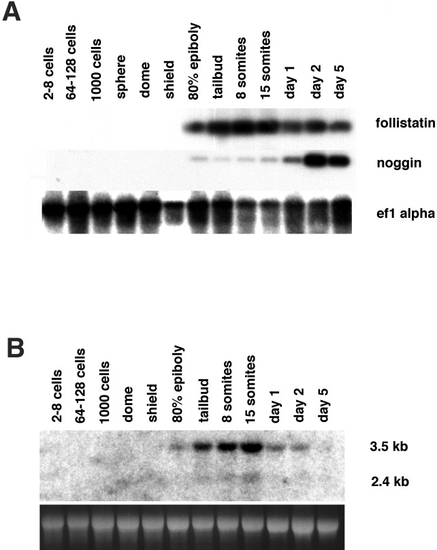
Temporal expression pattern of zebrafish follistatin and noggin. (A) Developmental RT-PCR analysis for follistatin, noggin, and the ef1α (control). Neither noggin nor follistatin transcripts are detected before 80% epiboly. noggin signals are weak during late gastrulation and segmentation stages, but much stronger during primordia stages, with a maximum at day 2. follistatin signals are of comparable strength throughout all investigated stages from 80% epiboly onward, with a slight maximum at the 8- and 15-somite stages. (B) Developmental Northern blot hybridized with follistatin probe. Ten micrograms of stage-specific total RNA was loaded per lane and stained with ethidium bromide as a loading control. Consistent with the results of RT-PCR, expression is first detectable at 80% epiboly, reaches a maximum at day 1 of development, and then declines. Note the two transcripts of follistatin. The larger transcript is 3.5 kb, the shorter transcript approximately 2.4 kb. |
|
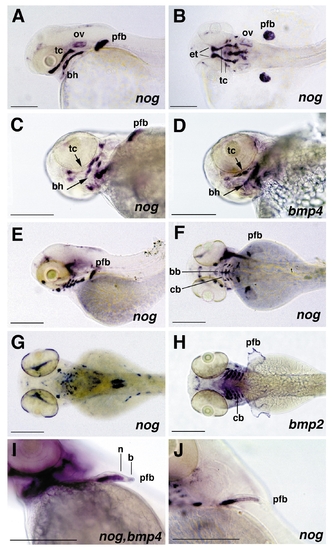
Expression pattern of noggin and bmp2b and bmp4 in branchial arches and pectoral fin primordia, revealed by whole mount in situ hybridization of albino zebrafish larvae. (A and B) 48 hpf, noggin expression, lateral view (A) and dorsal view (B) on head. noggin displays a broad expression in inner regions of the pectoral fin buds (pfb), while cells at the surface lack noggin expression. In addition, noggin is strongly expressed in the ethmoid plate (et) and the trabeculae cranii (tc), components of the neurocranium, and in specific subregions of the pharyngeal arches, including the anteriormost portion of the hyoid, the basihyale (bh). Weak staining is also observed in the otic vesicles (ov). (C and D) 60 hpf, noggin expression (C) and bmp4 expression (D), ventrolateral view on head; noggin and bmp4 are expressed in complementary patterns in subregions of the trabeculae cranii and the hyoid. noggin transcripts are present in distal regions of the tc and posterior regions of the hyoid, bmp4 transcripts in central regions of the tc (indicated with short arrows) and the bh (indicated with long arrows). (E and F) 72 hpf, noggin expression, lateral view (E) and dorsal view (F); noggin is expressed in the basibranchial (bb) and ceratobranchial (cb) components of the gill arches, while no expression is detected in the pharyngeal arches. (G and H) 84 hpf, noggin (G) and bmp2b (H) expression, dorsal view; noggin expression in the gill arches has declined, while bmp2b displays strong expression in the basibranchial components of the gill arches and in apical regions of the pectoral fin primordia. (I and J) noggin (I, J) and bmp4 (I) expression in pectoral fin primordia (pfb); (I) 60 hpf, (J) 72 hpf, anterolateral view; noggin (n) is expressed in proximal regions, bmp4 (b) in distal regions of the pectoral fin primordia (I). Expression of noggin in inner cells declines in a distal-to-proximal wave. Cells with reduced noggin levels display an elongated cell shape, which is characteristic for chondrocytes (J). Abbreviations: bb, basibranchial; bh, basihyale; cb, ceratobranchial; et, ethmoid plate; ov, otic vesicle; pfb, pectoral fin bud; tc, trabeculae cranii; hpf, hours after fertilization. |
|
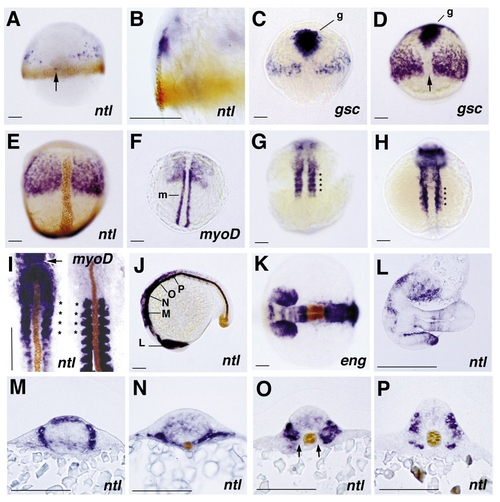
Expression pattern of follistatin in wild-type zebrafish embryos, revealed by whole-mount in situ hybridization. All embryos except the right embryo in (I) are stained for follistatin transcripts. In the case of double in situ hybridization immunostainings (A, B, E, I–K, M–P) and double in situ hybridizations (C, D, F), the second detected gene product is indicated in the lower right corner. (A and B) 60% epiboly, double stained for No tail protein (brown), dorsal view (A) and close-up of same embryo in dorsolateral view (B); scattered follistatin expression is seen in anterior paraxial regions and occasionally in single cells (indicated by an arrow, also in D) in posterior axial positions within the no tail expression domain in the notochord anlage (A). Expression of follistatin is restricted to involuted cells of the hypoblast (B). (C, D, and E) 80% epiboly (C), 90% epiboly (D), and tailbud stage (E), double stained for goosecoid (gsc) RNA (C, D, indicated with g) and No tail protein (E, brown), dorsal view; the paraxial follistatin expression domain grows, but expression remains excluded from anterior axial regions (E) which are characterized by the expression of the organizer-specific gene goosecoid (C, D). Posterior axial follistatin staining is weaker than staining in paraxial regions. In addition, the intensity of the axial follistatin staining varies in specimens from different in situ hybridizations (compare, e.g., C, D, and Fig. 7B). The reason for this variability is currently unknown. (F) 2-somite stage, double stained for myoD RNA (m), dorsal view; the posterior region of the follistatin expression domain and the anterior region of the myoD expression domain overlap, indicating that follistatin is expressed in the presumptive head mesoderm and a distinct anterior region of the presomitic mesoderm. The follistatin expression in the anterior presomitic mesoderm appears slightly condensed. (G and H) 5-somite stage (G) and 10-somite stage (H), dorsal view; the posterior paraxial follistatin expression is organized in a segmented pattern defining four stripes (indicated by asterisks) at identical anteroposterior positions in embryos of both stages. No additional stripes are visible in the 10-somite embryo. Note the intense anterior staining in (H), representing expression in the brain anlage. (I) 12-somite stage, stained for follistatin RNA and No tail protein (left) and for myoD RNA and No tail protein (right), dorsal view on spread embryos. In the left embryo, the anterior tip of the notochord anlage is indicated by an arrow. Comparison of the follistatin and myoD expression patterns assigns the intense segmented follistatin expression to the first 4 somites (indicated by asterisks). In addition, weaker follistatin expression is visible in somites 5 to 7. In all somites, follistatin expression is excluded from adaxial regions. (J) 20-somite stage, double stained for No tail protein, lateral view. Intense follistatin staining is observed in the head and anterior trunk region, weaker staining in more posterior somites. Indicated are the positions for which sections of similar embryos are shown in (L–P). (K) 20-somite stage, double stained for Engrailed protein; neuroectodermal follistatin expression is confined to the eye vesicles and four distinct bands in the forebrain, midbrain, and hindbrain, while the midbrain–hindbrain boundary region, which is characterized by the expression of engrailed, lacks follistatin transcripts. (L–O) Saggital (L) and transverse (M–P) 7-μm paraffin sections of doubly stained embryos of the 16-somite (M, L) and 25-somite (O, N, P) stage at the respective positions indicated in (J). (L) Section through the ventral (upper eye) and the medial (lower eye) part of the eye vesicles, revealing follistatin expression in posterior and distal regions of the eye primordia. (M) At the level of the anterior hindbrain, paraxial follistatin expression is restricted to an approximately three cell diameter broad region surrounding the neural tube. In the brain, follistatin is expressed in a distinct dorsal band and a more diffuse band in the ventral part of the tube. (N) More posteriorly, in the notochord region anterior of the somites, follistatin is expressed in the narrow band of cells located between the neural tube and the yolk and in ventral paraxial cells. (O) In the first 4, relatively small somites, follistatin is expressed throughout the entire somitic region except the adaxial positions adjacent to the notochord. Note the weak diffuse follistatin expression in the neural tube. (P) In posterior somites, follistatin expression is confined to ventral regions, which most likely represent the presumptive sclerotome, and to dorsal regions of the myotome in the vicinity of the neural tube. Abbreviations: eng, Engrailed; g, gsc,goosecoid; m, myoD, ntl, No tail. |
|
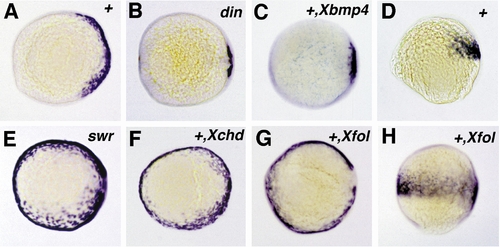
Expression of follistatin in chordino and bmp2b mutants and after overexpression of Xenopus follistatin,bmp4, or chordin. All embryos shown are at 90% epiboly and are viewed from the animal pole, except in (D and H), which are lateral views. Expression of follistatin is reduced in the chordino mutant embryo (B), spanning the dorsalmost 30° of the embryonic circumference, compared to 150° in wild-type sibling embryos (A). A similar dorsal retraction of the follistatin expression domain is obtained in wild-type embryos after injection of synthetic Xenopus bmp4 mRNA (C). In the bmp2b mutant embryo (E), the follistatin expression domain is expanded, spanning the entire embryonic circumference. The same effect is achieved in wild-type embryos by the injection of Xenopus chordin (F) and Xenopus follistatin mRNA (G). In (H), the Xfol-injected embryo of (G) is shown in a lateral view in comparison to an uninjected wild-type sibling embryo (D), showing that ectopic ventral zebrafish follistatin expression is restricted to the same anteroposterior levels like the wild-type expression on the dorsal side. Abbreviations: +, wildtype; din, chordino mutant dintt250; swr, bmp2b mutant swrta72. The second abbreviation in the upper right corner indicates the injected mRNA: Xbmp4, Xenopus bmp4; Xchd, Xenopus chordin; Xfol, Xenopus follistatin. |
|
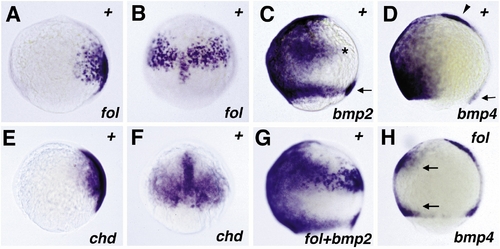
Comparison of zebrafish follistatin, chordino, bmp2b, and bmp4 expression (A–G) revealed by in situ hybridization and interaction between follistatin and bmp4 (H). All embryos are of the 80% epiboly stage. The in situ probes used are indicated in the lower right corner. (A and B) follistatin expression, lateral view (A), dorsal view (B). (E and F) chordino expression, (E) lateral view, (F) dorsal view; while chordino is expressed in organizer-derived cells in the presumptive prechordal plate, follistatin is expressed in more posterior regions of the notochord anlage. The paraxial chordino and follistatin expression domains in epiblast and hypoblast, respectively, are almost congruent, as confirmed by anti-Ntl counterstaining, marking the notochord anlage and the germ ring as reference points (not shown). At slightly later stages, the paraxial chordino expression domain appears slightly larger than the follistatin domain, extending more into marginal regions (not shown). (C) bmp2b expression, lateral view; the dorsal extension in the ventral expression domain is indicated by an asterisk, the expression in dorsal marginal cells by an arrow. (G) follistatin and bmp2b expression, lateral view. (D) bmp4 expression, lateral view; the expression domain in the dorsal marginal cells is indicated by an arrow, the expression domain in the presumptive prechordal plate by an arrowhead. (H) bmp4 expression after overexpression of zebrafish follistatin, lateral view. The region with reduced bmp4 expression is delimited by two arrows. |
Reprinted from Developmental Biology, 204, Bauer, H., Meier, A., Hild, M., Stachel, S., Economides, A., Hazelett, D., Harland, R.M., and Hammerschmidt, M., Follistatin and Noggin are excluded from the zebrafish organizer, 488-507, Copyright (1998) with permission from Elsevier. Full text @ Dev. Biol.