- Title
-
A role for FGF-8 in the dorsoventral patterning of the zebrafish gastrula
- Authors
- Fürthauer, M., Thisse, C., and Thisse, B.
- Source
- Full text @ Development
|
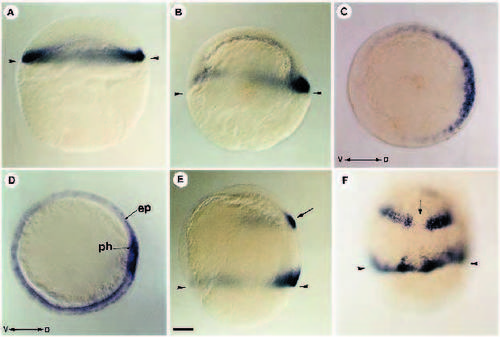
Localization of FGF-8 RNA during blastula and gastrula stages. (A) At blastula stage (30% epiboly), FGF-8 RNA is detected all around the margin of the blastoderm. (B) At early gastrula (50% epiboly) FGF-8 is restricted to the dorsal part of the marginal region. (C) Animal pole view of an embryo at 50% epiboly showing that fgf-8 is expressed as a dorsoventral gradient. (D) At midgastrula stage, FGF-8 transcripts are confined to the epiblast cells (ep) in contact with the underlying hypoblast. Dorsally, paraxial hypoblast (ph) territory is also positive for FGF-8 transcripts. (E) In addition to the marginal gradient, fgf-8 at midgastrula is expressed in the presumptive hindbrain (arrow). (F) Two bands of cells expressing FGF-8 are separated by a nonexpressing domain (arrow) which will later give rise to the ventral midline of the hindbrain. (A,B,E) Lateral views; (C) animal pole view; (D) vegetal pole view; (F) dorsal view. V, ventral; D, dorsal. Arrowheads in A,B,E,F indicate the position of the margin. Scale bar : 100 μm EXPRESSION / LABELING:
|
|
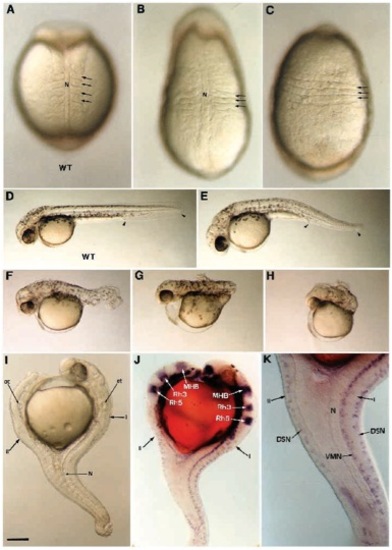
Morphological defects generated by ectopic expression of FGF-8. A range of dorsalization phenotypes is obtained after FGF-8 RNA injection. At the beginning of somitogenesis, the embryos display an elongated shape and somites (arrows) expand laterally (B) or even fuse ventrally (C) compared to wild-type control in A. At 36 hours, weak phenotype is apparent as a small reduction in the tail region delimited with arrowheads (E), compared to wild type in (D). Phenotype of intermediate strength are represented by a shortened and twisted tail (F). Strong phenotypes are characterized by a deletion of the tail (G) or by a deletion of both trunk and tail (H). (I-K) A 36 hours old dorsalized embryo presenting a double axis (I, primary axis; II, secondary axis). (I) Anteriorly the secondary axis forms opposite to the primary axis while both are fused in the caudal portion. (J) Using Krox20 (Oxtoby and Jowett, 1993) and Eng3 (Ekker et al., 1992) reveal that the secondary axis develops up to the posterior midbrain. Anterior to this position, forebrain, anterior midbrain, olfactory placodes and optic vesicules are missing in the secondary axis. (J,K) The secondary axis is defective in notochord and floor plate. Ventral motor neurons (VMN) are missing while dorsal sensory neurons (DSN) labeled with Isl- 1 (Inoue et al., 1994) are present. MHB, midbrain-hindbrain boundary labeled with Eng3; N, notochord; ot, otic vesicles, rh3, rh5, rhombomeres 3 and 5 labeled with Krox20. (A,B) Dorsal views; (C) ventral view; (D-K) lateral views. Scale bar, 100 μm in A-C,K; 250 μm in D-J. |
|
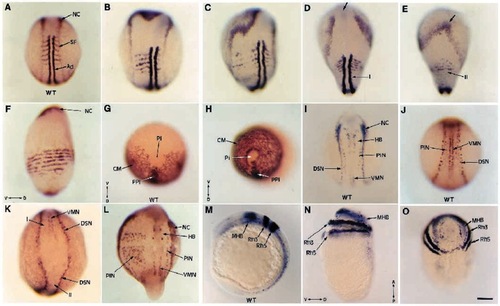
FGF-8 affects dorsoventral patterning of both mesoderm and ectoderm. After injection of FGF-8 RNA, embryos and their uninjected siblings were fixed at the beginning of somitogenesis, and probed using MyoD and AP2 in (A-F), snail2 (G,H), AS28 and AP2 (I-L), Krox20, Eng3 and AS28 in (M-O). Wild-type siblings in A,G,I,J,M). Labeling with MyoD and AP2 shows the gradual effects induced by FGF-8 from an enlarged paraxial territory (B) to a fragmented paraxial territory (C), or to the formation of a defective secondary axis on the ventral side of the embryo (E, compared to its dorsal wild-type side in D) or in extreme cases to a complete circularization of the paraxial territory (F). The cephalic mesoderm of these extreme dorsalized embryos, probed with snail2 (H compared to its wild-type control in G) is also circularized. The neural plate (the borders of which are labeled with AP2) is also expanded (B,C compared to A). When a secondary axis is formed (E), because of the absence of presumptive forebrain and midbrain, AP2 domain fuses anteriorly (arrow), while it remains separated in the primary axis (D). Analysis with AS28, Krox20 and Eng3 probes reveals the dorsolateralization effect of FGF-8 on the ectodermal layer. When a secondary axis (II) is formed as in K, dorsal sensory neurons (DSN) are present while ventral motor-neurons (VMN) are lacking. In embryos showing a dorsolateral expansion, as in L, the number of primary interneurons (PIN) on the affected side strongly increases (compared to the unaffected side or wild-type siblings in I,J). Strong dorsalized phenotypes (N,O) display a complete circularization of the neural plate visualized by Krox20 and Eng3 expression all around the yolk sac compared to wild-type control in (M). (A-D,I,J,L) Dorsal views; (E) ventral view; (G,H,O) animal pole views; (F,M,N) lateral views; (K) caudal view. (D,E) The dorsal and the ventral view of the same embryo. For references of the probes used, please see text. Ad, adaxial cells; CM, cephalic mesoderm; HB, hindbrain; MHB, midbrain-hindbrain boundary; NC, neural crest; Pi, pillow; PIN, primary interneurons; PPI, prechordal plate; Rh3, Rh5, rhombomeres 3 and 5; SF, somitic furrow; V, ventral; D, dorsal; A, anterior; P, posterior. Scale bar, 150 μm. |
|
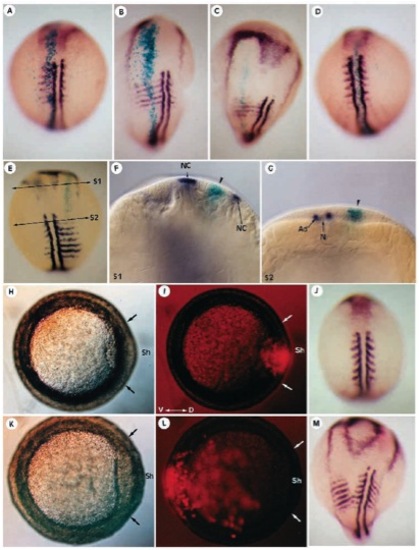
Correlation between the effect and localization of the clone secreting FGF-8. FGF-8 and β-galactosidase RNA were coinjected at the cleavage stage, and presence of β-galactosidase expression was revealed at early somitogenesis stages, using X-gal staining. The embryos then underwent in situ hybridization using MyoD and AP2 probes. b-galactosidase staining is always seen in the midline of the expanded dorsolateral territory resulting from misexpression of FGF-8 (A-C). When located in the axial midline (D), the FGF-8-secreting clone has no effect on the dorsoventral patterning of the embryo. (E) Dorsal view of an embryo displaying a partial secondary axis phenotype. Optical cross sections through plane S1 (F) or through S2 (G) showing that cells surrounding the FGF-8-secreting clone converge towards this position (arrowhead) instead of converging normally towards the dorsal side of the embryo. (G) The FGF-8 secreting cells are found in both mesoderm and ectoderm. (H,I) Embryo injected with FGF-8 and rhodamine coupled to 2 MDa dextran, visualized in bright field in H at gastrulation. (I) In this embryo, strong fluorescence is seen in the embryonic shield (Sh, delimited by two arrows). (J) In situ hybridization of the same embryo as in H,I using MyoD and AP2 probes at early somitogenesis showing that when located in the axial midline of the embryo at gastrulation, the FGF-8 clone has no effect on the dorsoventral patterning of the embryo. (K,L) When the FGF-8 clone is located opposite to the shield (Sh), the resulting phenotype consists of the formation of a secondary axis (M). (A-E,J,M) Dorsal views; (H,I,K,L) Animal pole views. Ad, Adaxial cells; N, notochord; NC, neural crest. |
|
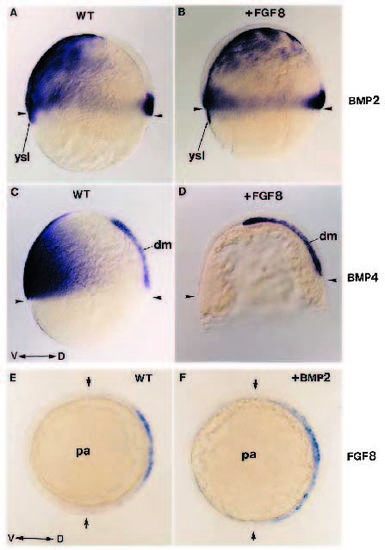
FGF-8 acts upstream of BMP2 and BMP4. (A-D) Expression of BMP2 and BMP4 at gastrula stage (60% epiboly) in wild type (A,C) and in embryos injected with FGF-8 RNA (B,D) showing that BMP labeling is absent from the ventral domain of the injected embryos. (A,B) BMP2 expression in wild type (A) and in FGF-8- injected embryos in B. (C,D) BMP4 expression in wild-type (C) and in FGF-8-injected embryos (D). (E,F) Expression at early gastrulation stage of FGF-8 in an embryo injected with BMP2 RNA (F) and its corresponding sibling (E). FGF8 expression is not affected by BMP2. (A-D) Lateral view; (E,F) animal pole view. dm, dorsal mesendoderm; pa, animal pole; ysl, yolk syncytial layer; WT, wild type; D, dorsal; V, ventral. Arrowheads in A-D show the position of the margin. Arrows in E-F indicate the limits of FGF8 gradient of expression. |