- Title
-
Developmental exposure to non-dioxin-like polychlorinated biphenyls promotes sensory deficits and disrupts dopaminergic and GABAergic signaling in zebrafish
- Authors
- Brun, N.R., Panlilio, J.M., Zhang, K., Zhao, Y., Ivashkin, E., Stegeman, J.J., Goldstone, J.V.
- Source
- Full text @ Commun Biol
|
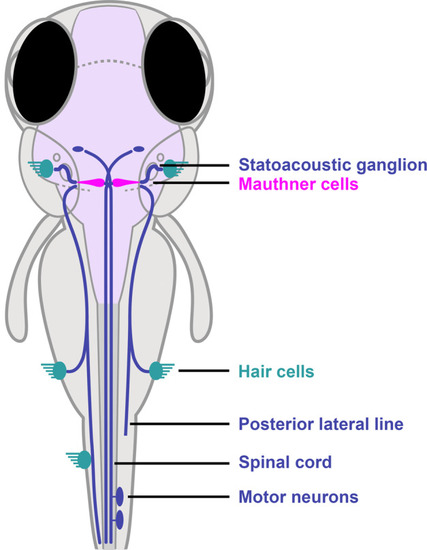
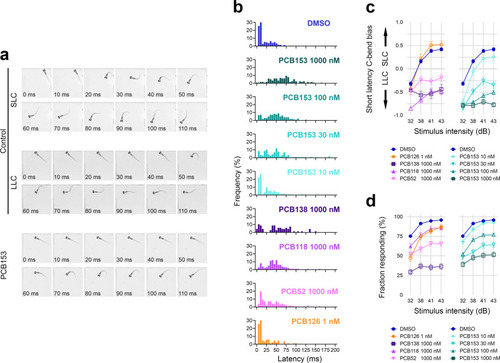
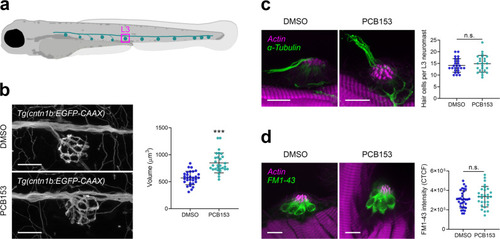
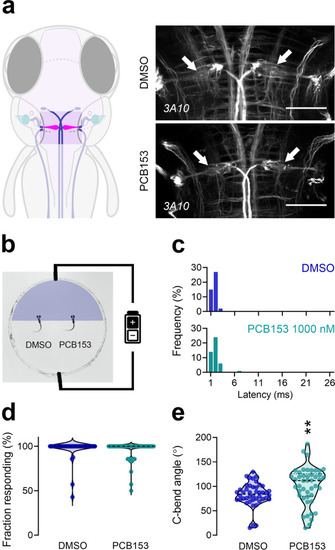
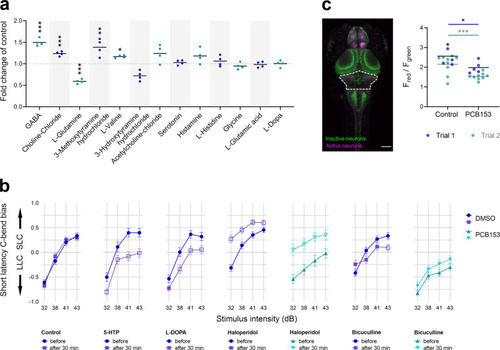
The proper execution of the auditory-evoked startle response relies on hair cells to sense the auditory stimulus, Mauthner cells to integrate the signals, and motor neurons and musculature to execute the startle behavior. |
|
|
|
|
|
|
|
|