- Title
-
npc2-Deficient Zebrafish Reproduce Neurological and Inflammatory Symptoms of Niemann-Pick Type C Disease
- Authors
- Wiweger, M., Majewski, L., Adamek-Urbanska, D., Wasilewska, I., Kuznicki, J.
- Source
- Full text @ Front. Cell. Neurosci.
|
Zebrafish |
|
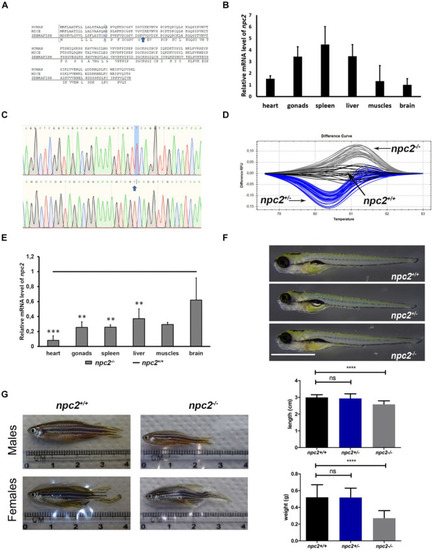
Nile blue staining distinguishes PHENOTYPE:
|
|
Specificity of Nile blue staining in the olfactory organ in PHENOTYPE:
|
|
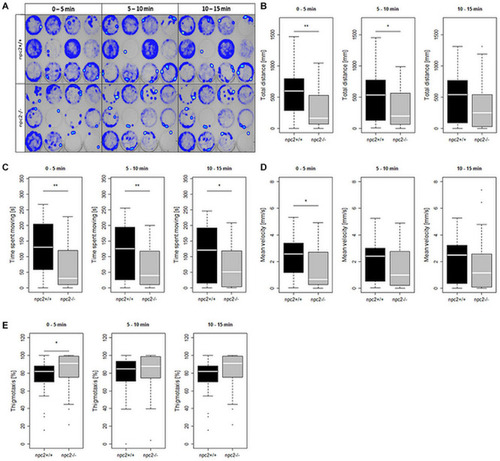
Decrease in mobility and increase in thigmotaxis in PHENOTYPE:
|
|
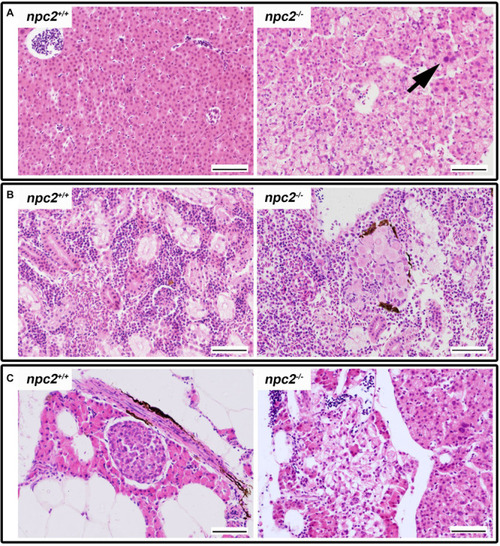
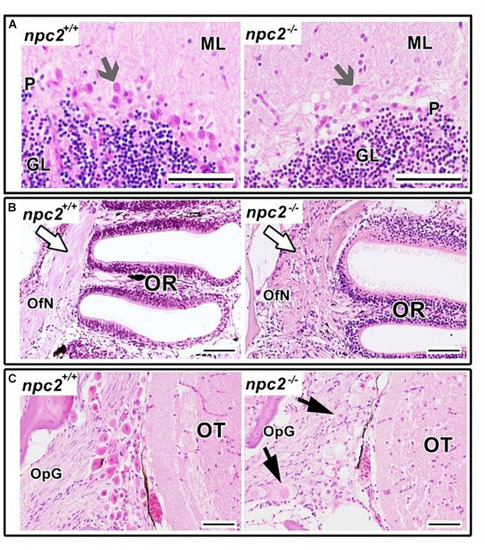
Pathological changes in soft tissues in |
|
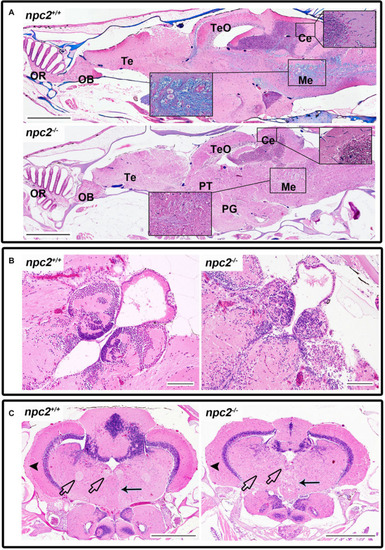
Pathological changes in the central nervous system in adult npc2–/– zebrafish. (A) Luxol fast blue staining showed differences in myelination between wildtype and npc2–/– fish. (B) Changes in habenula structure were present in some npc2–/– fish, which lacked the characteristic structure of this part of the habenular tract. (C) Midbrain with vacuolization of the habenula tract (blue arrow) with fascicular retroflexus (blue arrowhead) and degenerative changes in the medial and lateral longitudinal fascicle. OB, olfactory bulb; OR, olfactory rosette; Te, telencephalon; TeO, tectum opticum; Ce, cerebellum; Me, medulla; PG, preglomerular complex; and PT, posterior tuberculum. (A) LFB staining. Scale bar = 500 μm. (B,C) H&E staining. Scale bar = 100 μm. (C) scale bar PHENOTYPE:
|
|
Pathological changes in PHENOTYPE:
|
|
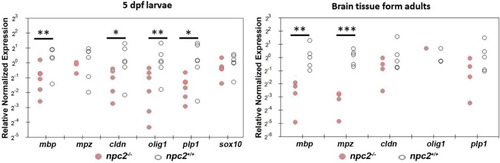
Decrease in myelination in |
|
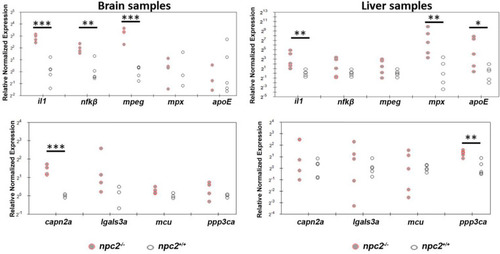
Signs of inflammation and alterations of Ca2+ homeostasis in |
|
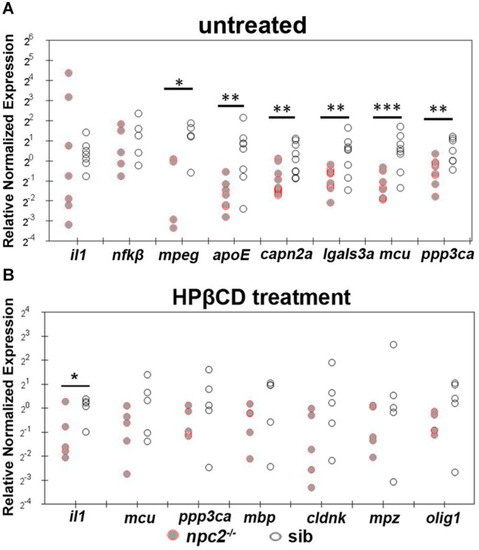
Treatment with 2-hydroxypropyl-β-cyclodextrin increased the expression of genes that are related to inflammation and Ca2+ homeostasis in 5 dpf |