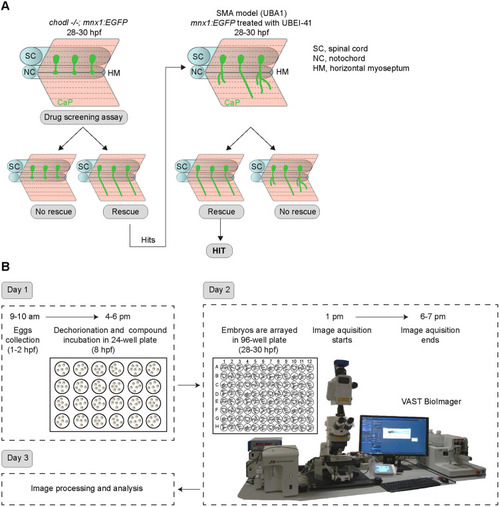
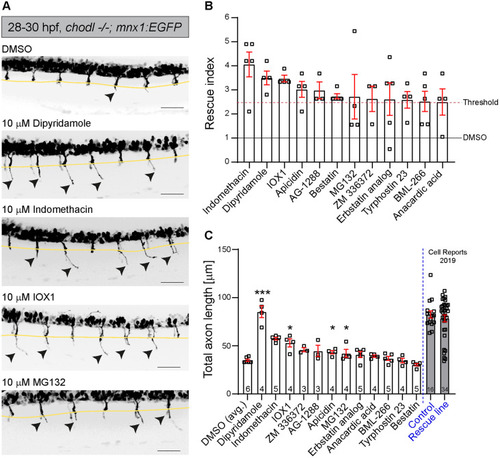
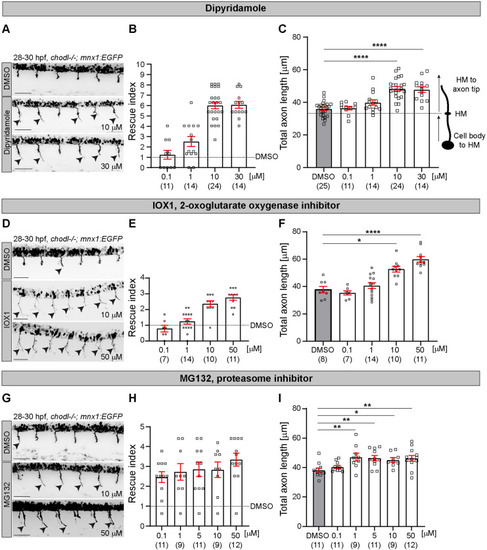
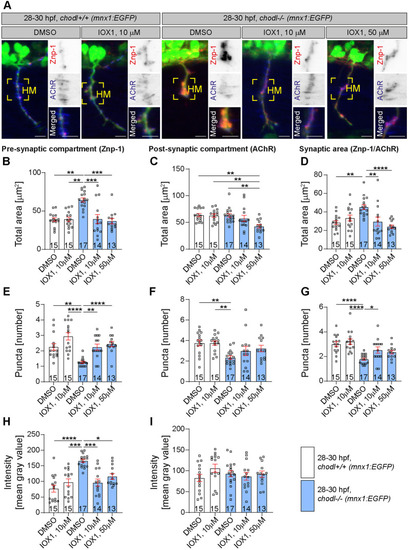
- Title
-
Automated in vivo drug screen in zebrafish identifies synapse-stabilising drugs with relevance to spinal muscular atrophy
- Authors
- Oprişoreanu, A.M., Smith, H.L., Krix, S., Chaytow, H., Carragher, N.O., Gillingwater, T.H., Becker, C.G., Becker, T.
- Source
- Full text @ Dis. Model. Mech.
|
|
|
EXPRESSION / LABELING:
PHENOTYPE:
|
|
|
|
|
|
|
|
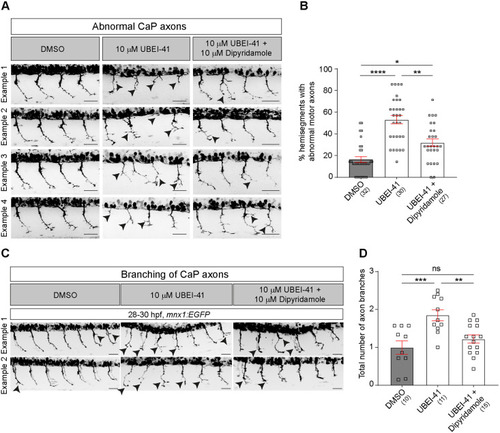
(A) Representative images of the lateral trunk of 28-30 hpf wild-type embryos. Abnormal motor neurons, i.e. short, missing or branched axons, are indicated by arrowheads. (B) The percentage of abnormal motor axons is decreased in embryos after combined treatment with UBEI-41 and dipyridamole (10 µM each) compared to those treated with UBEI-41 alone (Kruskal–Wallis test ****P<0.0001 with Dunn's multiple comparison test: ****P<0.0001, **P=0.0078 *P=0.0124, statistical power=0.999). (C) Representative images of 28-30 hpf wild-type embryos treated with DMSO, UBEI-41 or UBEI-41 and dipyridamole are shown. Abnormal axon branching is indicated by black arrowheads. Scale bars: 50 µm. (D) Quantification of axonal branching only. The number of axon branches is increased in embryos after treatment with dipyridamole (10 µM) compared to those treated with DMSO (control) (one-way ANOVA test ***P=0.0006 with Tukey's multiple comparison test: ***P=0.0007, **P=0.0062 statistical power=0.9618). All scale bars: 50 µm. Each data point represents one animal and n numbers are indicated in parenthesis. Error bars represent means±s.e.m. |
|
|