- Title
-
Involvement of cerebellar neural circuits in active avoidance conditioning in zebrafish
- Authors
- Koyama, W., Hosomi, R., Matsuda, K., Kawakami, K., Hibi, M., Shimizu, T.
- Source
- Full text @ eNeuro
|
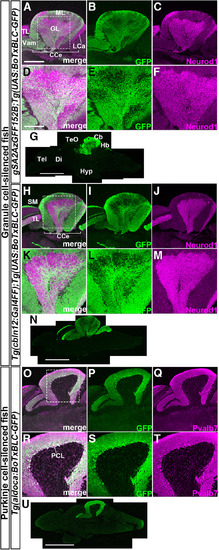
Establishment of Tg fish that express botulinum toxin in GCs or PCs. Sagittal sections of adult |
|
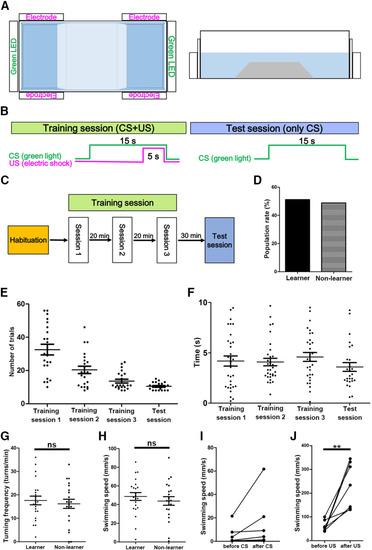
Active avoidance conditioning of wild-type fish. |
|
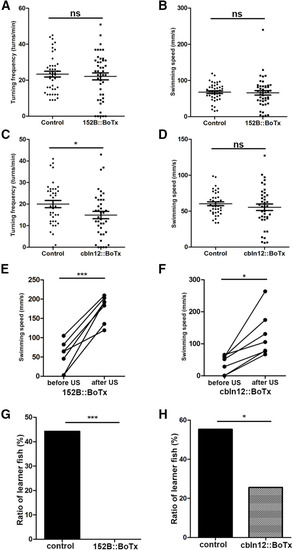
Expression of botulinum toxin in GCs suppresses active avoidance conditioning. |
|
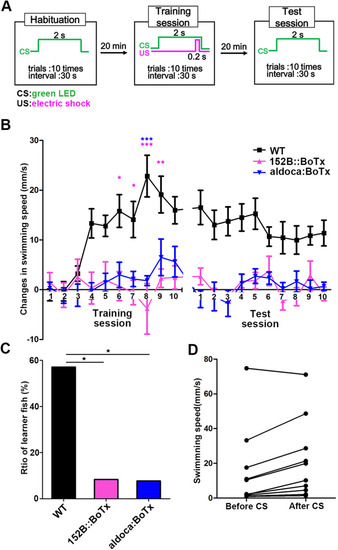
Expression of botulinum toxin in PCs suppresses active avoidance conditioning. |
|
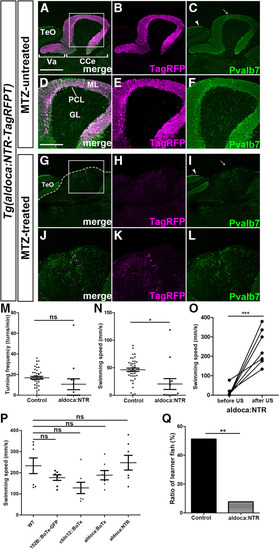
NTR-mediated ablation of PCs in adult fish suppresses active avoidance conditioning. |
|
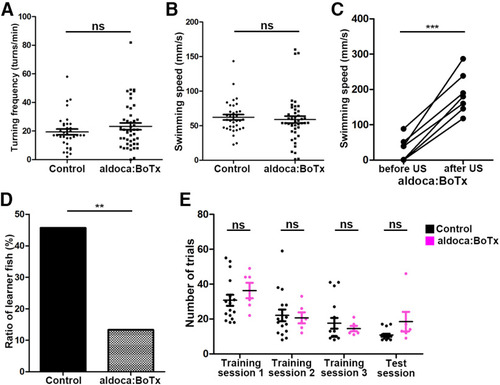
Expression of botulinum toxin in GCs or PCs also perturbs classical conditioning responses. |