- Title
-
The endocannabinoid system and retinoic acid signaling combine to influence bone growth
- Authors
- Fraher, D., Mann, R.J., Dubuisson, M.J., Ellis, M.K., Yu, T., Walder, K., Ward, A.C., Winkler, C., Gibert, Y.
- Source
- Full text @ Mol. Cell. Endocrinol.
|
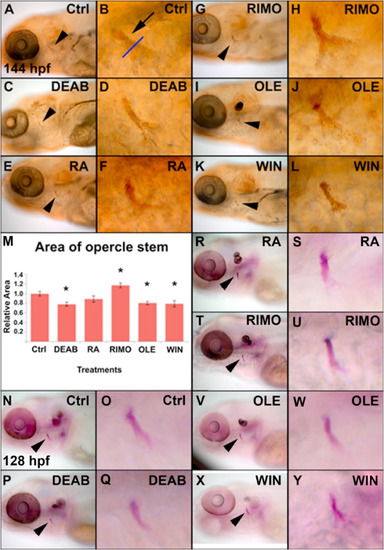
Modulation of the retinoic acid and endocannabinoid signaling pathways influence larval bone mineralization and calcification. Zebrafish were treated from 50 to 72 hpf with DEAB, RA, RIMO, OLE, and WIN and mineralization of the opercle (arrowhead) was detected at 144 hpf with von Kossa stain (A–L). Mineralization of the opercle stem was quantified (M, n = 10) from the area of labeling above the opercle fan, marked by the blue line (B, arrow) (See Supplemental Fig. 1 for a schematic representation of the positioning of the blue line in the opercle). Zebrafish embryos were treated from 50 to 72 hpf and stained with alizarin red at 128 hpf to detect calcification (N–Y, n = 10). The effects on calcification from DEAB, RA, RIMO, OLE, and WIN treatments are consistent with the effects on mineralization. Imaged zebrafish are a representation of zebrafish in their respective groups. |
|
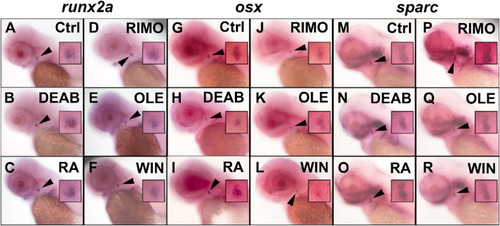
Early, intermediate and late bone-specific gene expression is altered by modifying RA and ECS signaling in zebrafish. Zebrafish embryos were treated with DEAB, RA, RIMO, OLE, and WIN from 36 to 50 hpf. At 50 hpf runx2a expression was assessed by WISH (A–F). Zebrafish embryos were treated between 36 and 62 hpf and at 62 hpf osx expression was assessed (G–L). Zebrafish embryos were treated between 36 and 72 hpf and sparc expression was assessed at 72 hpf (M–R). DEAB treatment increased runx2a expression (B) and decreased osx and sparc (H,N) while RA (C) treatment decreased runx2a and increased osx and sparc (I,O). RIMO treatment increased the expression of each gene (D,J,P) while both OLE and WIN decreased the expression of each (E,F,K,L,Q,R). n = 10 for all treatments and WISH analyses. Opercle insets are 4X magnifications of the original photos. |
|
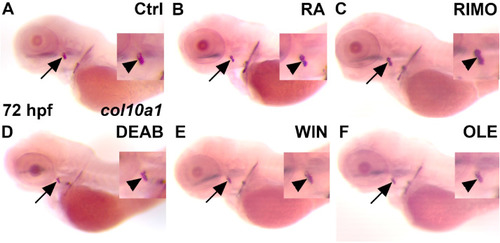
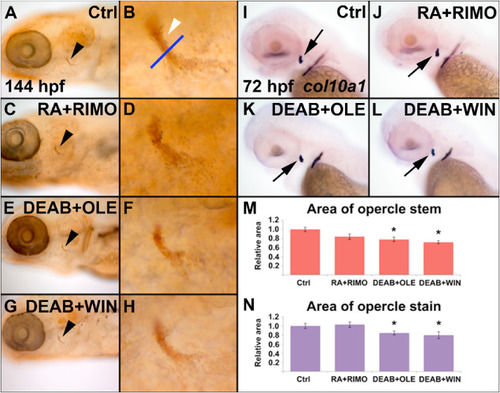
RA and ECS signaling alter col10a1 expression in zebrafish. Zebrafish were treated between 56 and 72 hpf for RA (B) and 48–72 hpf with RIMO (C), DEAB (D), WIN (E), and OLE (F). At 72 hpf, WISH was performed to observe any changes in col10a1 expression (B–F) as compared to the control (A) at the opercle marked with a black arrow, or marked by an arrowhead in close-ups. RA (B) and RIMO (C) treatments increased col10a1 expression as compared to control (A). DEAB (D), WIN (E) and OLE (F) treatments decreased col10a1 expression as compared to control (A). n = 10 for each treatment group. |
|
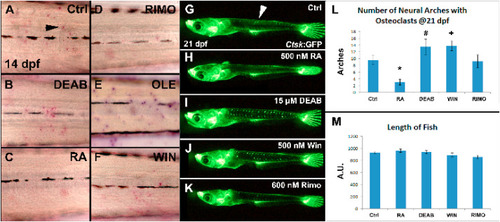
RA signaling inhibits and the ECS enhances osteoclast development. Zebrafish larvae were treated between 12 and 14 dpf with DEAB, RA, RIMO, OLE, and WIN and active osteoclasts were stained at 14 dpf with TRAP (A–F). DEAB or OLE treatment increased TRAP labeled osteoclasts (B,E), whereas RA or RIMO decreased stained cells (C,D). A medaka line with a cathepsin k promoter driving GFP was used to assess osteoclast development in neural arches at 21 dpf following 2 days of treatment (G–L). RA treatment decreased detectable osteoclasts (H,L). DEAB or WIN treatment increased osteoclast abundance (I,J,L). RIMO treatment showed no effect on osteoclasts (K,L). Length of fish, as a measure of developmental defects from treatments, was not different between treatment groups and controls (M). *<0.001, # = 0.153, + = 0.053. n = 8 for TRAP staining, n = 8–12 for neural arch assessments, n = 3–6 for length measurements. |
|
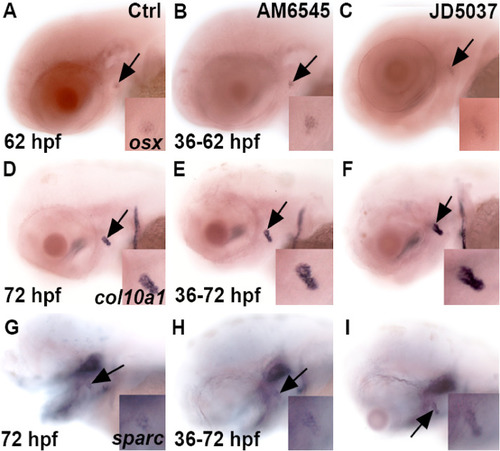
M and JD effects on col10a1 expression in mid-to-late time development. Shows the effects of AM6545 and JD5037 on gene expression for 62 (A,B,C) and 72 hpf (D–I) at the opercle bone marked with a black arrow. Zebrafish were exposed to the compounds from 36 to 62 hpf for osx (B,C) and 36–72 hpf for col10a1 and sparc, respectively. AM6545 (B) and JD5037 (C) increased expression of osx at 62 hpf as compared to the control (A) at 62 hpf. Both compounds (E,F) showed an increase in col10a1 expression at 72 hpf as compared to the control (D) at 72 hpf. Additionally, AM6545 (H) and JD5037 (I) increased sparc expression as compared to the control (G) at 72 hpf. n = 10 for each treatment group. For each treatment, 100% of zebrafish (B,C,E,F,H,I) showed an increase in gene expression as compared to their control (A,D,G) regardless of the gene being observed. |
|
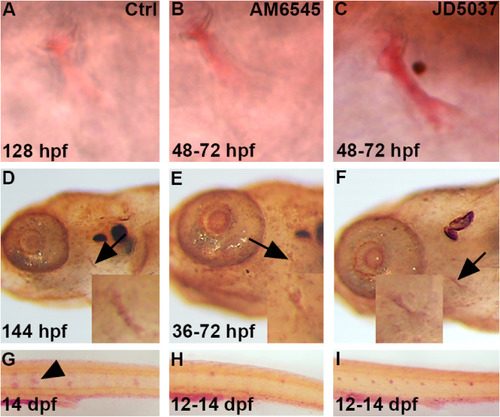
Blocking the peripheral activity of CB1 increases bone calcification and mineralization while decreasing osteoclast activity. Zebrafish were exposed to either AM6545 (B) or JD5037 (C) from 48 to 72 hpf. Alazarin red staining was performed at 128 hpf to determine if there were any changes in calcification at the opercle bone, marked by the black arrows, after exposure (B,C) as compared to control (A). Both AM6545 (B) and JD5037 (C) showed a calcification increase as compared to the control (A) at 128 hpf. n = 12 for each treatment group. For AM6545 (B), 100% of zebrafish showed a significant increase in calcification as compared to the control (A). For JD5037 (C), 100% of zebrafish showed a significant increase in calcification as compared to both the control (A) and AM6545 (B). Zebrafish were exposed to AM6545 (E) and JD5037 (F) from 36 to 72 hpf and fixed at 144 hpf. At 144 hpf, Von Kossa staining was performed in order to observe any changes in mineralization after exposure (E,F) as compared to the control (D). Images were taken at 8x and 20x (D,E,F). Both AM6545 (E) and JD5037 (F) exposure led to a mineralization increase at 144 hpf as compared to the control (D) at 80x and 200x magnification. n = 10 for each treatment group. 100% of AM6545 (E) exposed zebrafish showed a significant increase mineralization as compared to the control (D). As for JD5037 (F), 100% of zebrafish showed a significant mineralization increase as compared to the control (D), and the AM6545 (E) exposed zebrafish. Tartrate Resistant Acid Phosphatase (T.R.A.P.) stain shows presence of osteoclasts. The zebrafish were exposed to AM6545 (H) and JD5037 (I) from 12 to 14 dpf. At 14 dpf, the larvae were fixed and TRAP was performed to determine any changes in osteoclast presence (G,H,I). Both AM6545 (H) and JD5037 (I) exposure showed a significant decrease in osteoclast presence at 14 dpf as compared to the control (G) at 14 dpf. n = 10 for each treatment group. For the control group (G) 100% showed high osteoclast activity that is common at 14 dpf. For AM6545 (H), 70% showed little to no osteoclast activity, and 30% showed a significant decrease in osteoclast presence as compared to the control. For JD5037 (I), 80% showed little to no osteoclast presence, and 20% showed a significant decrease in osteoclast presence. Overall, after exposure to either compound (H,I), 100% of the zebrafish showed a significant decrease in osteoclast presence as compared to the control (G). |
|
Fig. 7. Combined sup-optimal doses of RA and ECS modulating chemicals is sufficient to influence bone development. Zebrafish were treated from 50 to 72 hpf with 60% combined doses of RA + RIMO, DEAB + OLE, or DEAB + WIN and mineralization of the opercle (black arrowhead) and opercle stem (white arrowhead) was assessed at 144 hpf with von Kossa stain (A–H). RA + RIMO showed no effect on mineralization (C,D). Both DEAB + OLE or DEAB + WIN treatments were sufficient to reduce mineralization (E–H). Measurements of the area of staining of the opercle stem support these observations (M, n = 9). The effects of combined doses on col10a1 expression was assessed at 72 hpf following treatment from 36 to 72 hpf (I–L). RA + RIMO showed no effect on col10a1 expression (J). Both DEAB + OLE or DEAB + WIN treatments were sufficient to reduce expression (K,L). Quantification of staining of the opercle supports the observed expression (N, n = 10). |
|
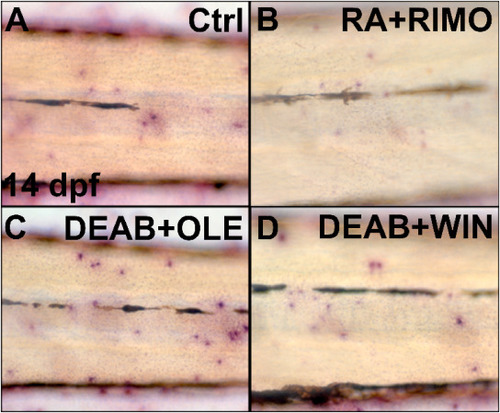
Combined sup-optimal doses of RA and ECS modulating chemicals influence osteoclast activity. Zebrafish were treated from 12 to 14 dpf with 60% combined doses of RA + RIMO, DEAB + OLE, or DEAB + WIN and osteoclast activity was assessed with TRAP stain at 14 dpf (A–D). RA + RIMO treated zebrafish had reduced numbers of labeled osteoclasts compared with controls (A,B). DEAB + OLE or DEAB + WIN had increased stained osteoclasts (C,D). n = 8 for each treatment group. (Fig. 8C and D). These results demonstrate that combined modulations of RA and ECS signaling have quantifiable effect on the mineralization of bone, the formation of osteoblasts and the number of active osteoclasts in vivo. |
Reprinted from Molecular and Cellular Endocrinology, 529, Fraher, D., Mann, R.J., Dubuisson, M.J., Ellis, M.K., Yu, T., Walder, K., Ward, A.C., Winkler, C., Gibert, Y., The endocannabinoid system and retinoic acid signaling combine to influence bone growth, 111267, Copyright (2021) with permission from Elsevier. Full text @ Mol. Cell. Endocrinol.