- Title
-
Semaphorin 3fa Controls Ocular Vascularization From the Embryo Through to the Adult
- Authors
- Halabi, R., Watterston, C., Hehr, C.L., Mori-Kreiner, R., Childs, S.J., McFarlane, S.
- Source
- Full text @ Invest. Ophthalmol. Vis. Sci.
|
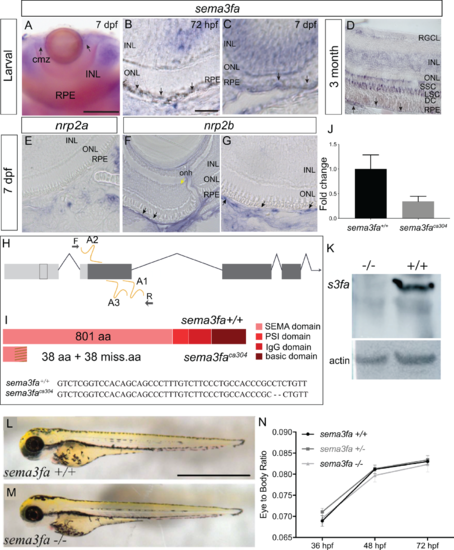
Sema3fa is expressed by the RPE and inner retina of larval and adult zebrafish. (A–D) sema3fa RNA ISH in whole mount (A) and retinal sections (B–D). ISH signal is detected in the retinal pigment epithelium (RPE) during development (A–C) and into adulthood (D). In larval and adult retina, sema3fa is also expressed strongly in the inner nuclear layer (INL) and CMZ, with expression in the outer nuclear layer (ONL) observed in the adult. (E–G) Expression at 7 dpf of mRNA for the known Sema3fa receptor nrp2b (F,G) but not nrp2a (E) by the endothelial cells of the retinal artery that runs alongside the optic nerve head (yellow arrow) and the choroid vessels lining the back of the eye (black arrows). Note that to minimize the pigment of the RPE, retinal sections were either from larvae treated at 24 hpf with 1-phenyl-2-thiourea (A–C, E–G), or were bleached (D). (H) Chromosomal overview of the sema3fa locus targeted by CRISPR/Cas9 mutagenesis to exon 1 (guide RNA: A1-3) and primers used to identify the mutation. UTR: untranslated region; F: forward primer; R: reverse primer. (I) Schematic representation of WT and premature stop codon mutant proteins. sema3faca304 fish have a 2 bp deletion (dashes) and produce a predicted product of 76 aa. Dashes represent missense amino acids (miss.aa). (J) RT-qPCR of sema3fa mRNA levels in WT and sema3fbca304 embryos at 48 hpf suggest nonsense mediated decay of mRNA transcript (N = 3). Error bar represents standard error of the mean (SEM). (K) Western blot of protein isolated from 5-6 dpf WT and sema3faca304 mutant fish processed with a custom-made antibody against zebrafish Sema3fa. The antibody recognizes a protein of the appropriate size for Sema3fa in WT fish, which is absent in the mutants. Loading control is ß-actin. (L, M) Lateral views of 72 hpf WT (H) and sema3faca304 (I) fish. (N) The ratio of eye width to anterior-posterior body length, measured along the antero-posterior axis, at 36, 48, and 72 hpf, arguing that there are no gross abnormalities in body or eye growth in the sema3fa heterozygote (n = 10) and homozygote (n = 4) mutant embryos as compared to WT (n = 10). Scale bars: A: 100 µm; B: 15 µm; L: 1 mm. |
|
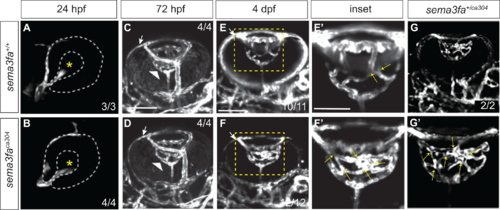
Sema3fa is required to refine and limit retinal vessel branching in the larval eye. Live imaging of vessels (white) in Tg(kdrl:mCherry) WT (A,C,E,E’) and sema3faca304 heterozygous (G,G’), and homozygous (B,D,F,F’) eyes during retinal intraocular vascular development acquired by confocal microscopy. (A, B) In lateral views of WT (A) and sema3faca304 homozygous embryos, the hyaloid artery (endothelial cells labeled by mCherry) contacts the lens (asterisk) of the retina by 24 hpf (N = 1 WT n = 3; sema3faca304 n = 4). (C, D) Ventral views of WT (C: N = 1, n = 4) and sema3faca304 (D: N = 1, n = 4) eyes reveal that the hyaloid artery (arrowhead) forms a vascular plexus network around the lens by 72 hpf. Also evident is the nasal ciliary artery (arrows). (E–G) The WT intraocular hyaloid vessel (E’ is a magnified view of the boxed area in E) undergoes remodeling between 4-5 dpf to simplify the network (Hartsock et al., 2014). At 4 dpf, the numbers of vessel crossovers (arrows) are increased in sema3fa heterozygous (G,G’: N = 1, n = 2/2) and homozygous (F,F’: N = 4, n = 12/12) mutant embryos as compared to WT siblings (E, E’: N = 4, n = 10/11 show refinement). Scale bar: 100 µm; 50 µm for E’–G’. |
|
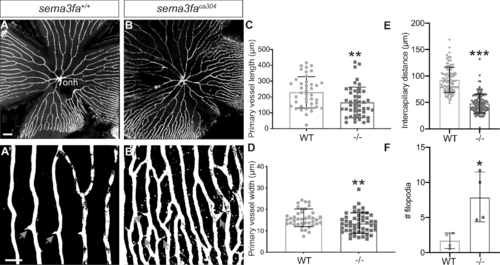
Increased vascularization of the retina is observed into adulthood. (A, B) Vessels (white) in retinal flat mounts of Tg(kdrl:mCherry) WT siblings (A) and sema3faca304 (B) 5 month fish. Capillaries furthest from the optic nerve head (onh) in the peripheral retina are shown in A’,B’. A greater number of filopodial interconnections between capillaries are present in mutant retina (gray arrows). (C) Primary vessel length as measured from the optic nerve head (onh) to the first branching event is significantly shorter in mutants (**P = 0.0016, N = 2, n = 6 retinas) as compared to WT siblings (N = 2, n = 6 retinas). (D) The average width of the primary vessels is reduced significantly in mutants (**P = 0.0078; N = 2, n = 6 retinas) as compared to WT (N = 2, n = 6 retinas). (E) Measurement of the intercapillary distance shows that mutant capillaries are more closely spaced (n = 4 retinas, P < 0.0001) than in WT (n = 4 retinas). (F) Average number of filopodia in a region of interest in the retinal periphery (WT n = 4; sema3faca304 n = 4; P = 0.029). Error bars represent standard deviation (SD). Statistics represent the nonparametric Mann-Whitney U test. Scale bar: 200 µm (A) and 50 µm (A’). |
|
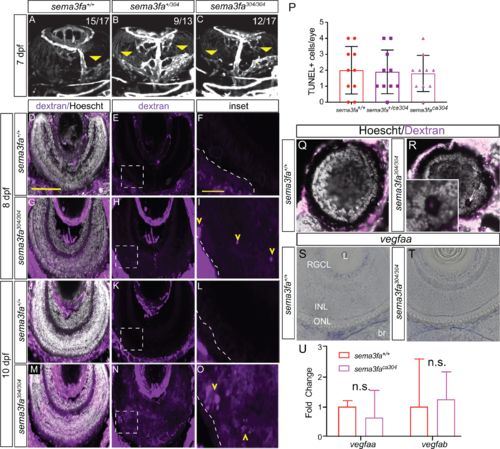
The sema3fa-deficient embryos present with leaky vessels within the outer retina. (A–C) Vessels (white) in ventral views of the eyes of live imaged Tg(kdrl:mCherry) 7 dpf larvae. In WT (A: N = 6, n = 15/17) larvae the extraocular choroid plexus (yellow arrowhead) has begun to branch over the back of the retina. Heterozygous (B: N = 4, n = 9/13) and homozygous (C: N = 6, n = 12/17) semafaca304 eyes exhibit a dramatic increase in the choroid vasculature as compared to WT. (D–O) Sema3fa deficiency permits the entry of leaky vessels into the neural retina. Thick (40 µm) cryosections through the eyes of embryos injected at 8 dpf (E–I) or 10 dpf (J–O) with 2,000,000 MW rhodamine dextran and fixed 24 hours later. Sections are counterstained with nuclear label (Hoechst). Blood vessels (yellow arrowheads) infiltrate aberrantly into the outer retina of homozygous embryos at 8 dpf (I: N = 2, n = 4/4) and 10 dpf (O: N = 3, n = 10/10), but are not present in WT embryos (F: N = 2, n = 4/4 for 8 dpf; L: N = 2, n = 10/10 for 10 dpf). Dextran is present in the neural retina at 8 and 10 dpf in homozygous mutant eyes (H, I: 8 dpf, N = 2, n = 4/4; N, O: 10 dpf, N = 3, n = 8/10), but is essentially absent from WT (E, F: 8 dpf, N = 2, n = 4/4; K, L: 10 dpf, N = 3, n = 9/10) eyes. Dashed line represents the boundary between the RPE and neural retina. Boxed areas in E, H, K, and N are shown magnified in F, I, L, and O. (P) Average numbers of TUNEL+ apoptotic cells in whole mount WT, heterozygous, and homozygote eyes at 7 dpf (P > 0.99; N = 2, n = 10 for each genotype). (Q, R) An example of the peripheral retina of a WT (Q) and semafaca304 (R) 10 dpf larvae, where penetrating pathologic vessels in the mutant appear to disrupt retinal cellular architecture (arrow). (S,T) vegfaa expression in plastic sections of WT (S) and sema3fa mutant (T) 7 dpf retinas. (U) RT-qPCR for vegfaa and vegfab performed on mRNA isolated from WT (n = 5) and semafaca304 (n = 5) 7 dpf eyes. br, brain; INL, inner nuclear layer; L, lens; ONL, outer nuclear layer; RGCL, retinal ganglion cell layer. Error bars are SD. Scale bar: D: 100 µm; F, I, L, O: 20 µm. |
|
Vessel infiltration persists in the adult sema3fa mutant retina. (A–H) Cryosections made through the retinas of Tg(kdrl:mCherry) sema3fa WT (A–C) and mutant (D–F) 5 month old siblings. Nuclei are labeled by Hoechst (white). Blood vessels were detected in the area of the photoreceptor outer segments (os) in the majority of semafaca304 eyes (N = 1, n = 3/4), but not in WT retinas (N = 1, n = 4). C and F are magnified views of the boxed regions in B and E. Yellow chevron points to blood vessel. (G, H) One-year-old adult sema3fa+/+ (G) and sema3fa−/− (H) fish were injected with 2,000,000 MW rhodamine dextran (purple) and fixed 4 hours later. Thick (40 µm) eye cryosections counterstained with nuclear label (Hoechst) reveal leakage of dye (purple, yellow chevron) in the neural retina of semafaca304 fish (H: n = 3/5), but not WT siblings (G: n = 3/3). inl, inner nuclear layer; onl, outer nuclear layer; RGC, retinal ganglion cell layer; RPE/C, retinal pigment epithelium/choroid. Scale bar: 50 µm. Scale bar of inset: 10 µm. |
|
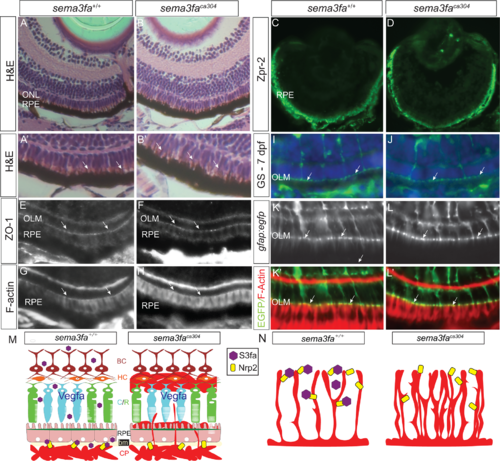
No obvious disruption of the RPE and outer limiting membrane with the loss of Sema3fa. (A, B) Transverse sections of 8 dpf WT and sema3faca304 mutant light-adapted retinas labeled with hematoxylin and eosin. A higher power view of the RPE is shown in A’ and B’. No obvious gaps are seen in the RPE, and apical microvilli (arrows) are evident in both genotypes (N = 3, n = 9 at 72 hpf for both genotypes; N = 2, n = 10 at 8 dpf for both genotypes). (C, D) Immunolabeling of the RPE with the Zpr2 antibody is comparable in WT (N = 2, n = 9) and mutant (N = 2, n = 9) retinas. (E–H) No obvious disruption of the outer limiting membrane (OLM; arrows), as stained with an antibody for the adhesive junction protein ZO-1 (N = 2, n = 10/10 WT eyes; n = 7/7 sema3faca304 eyes) and phalloidin that labels F-actin (N = 3, n = 15/15 WT eyes; n = 16/16 sema3faca304 eyes). (I, J) Glutamine synthetase immunolabeling of the Müller glia in WT (I; N = 2, n = 11/11) and mutant (J: N = 2, n = 10/10) 7 dpf retinas, revealing labeling of the apical Müller glia endfeet at the OLM (arrows) in both genotypes. (K, L) Labeling at the OLM (arrows) of F-actin (phalloidin) and Müller glia endfeet in a Tg(gfap:egfp) background in WT (n = 7/7) and sema3faca304 (n = 8/8) 15 dpf retinas. (M, N) Working model of roles for Sema3fa in regulating vascularization of the neural retina. Choroid vasculature (M): In WT, the choroid plexus (CP) remains outside of the RPE and Bruch's membrane (bm) through the release of Sema3fa (purple hexagon) by cells of the inner nuclear layer and the RPE. If Sema3fa signaling is perturbed, vessels pass through the RPE and leak into the nuclear layers containing the cone and rod (C/R) photoreceptors, horizontal cells (HC), and bipolar cells (BC). Hyaloid/retinal vasculature (N): Sema3fa (purple hexagon) maintains spacing and reduces the density of the blood vessel networks in WT retinas. In the absence of Sema3fa blood vessels overgrow. We propose that in both models Sema3fa acts via Nrp2b (yellow rectangle) and blood vessel growth is supported but not driven by the pro-angiogenic Vegf. EXPRESSION / LABELING:
PHENOTYPE:
|