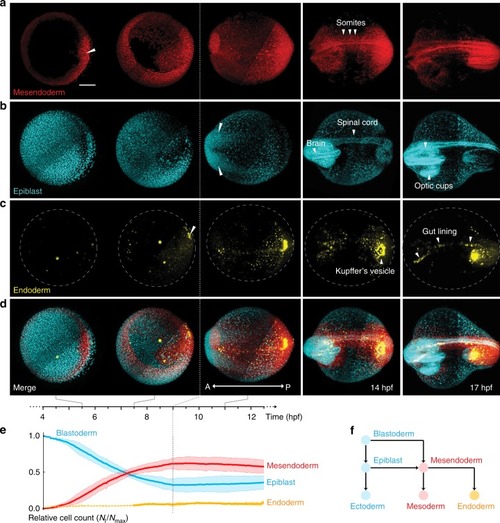
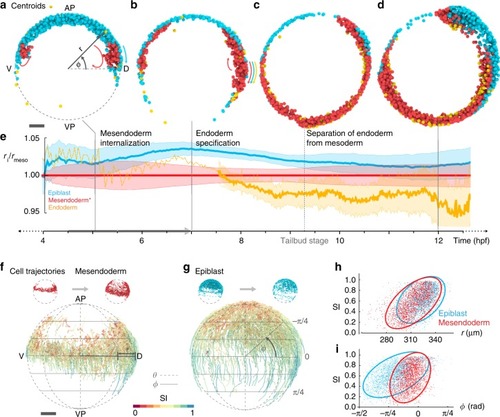
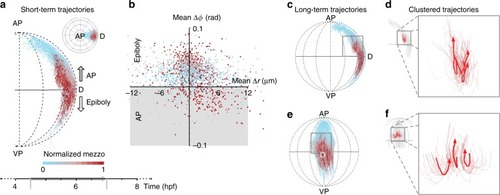
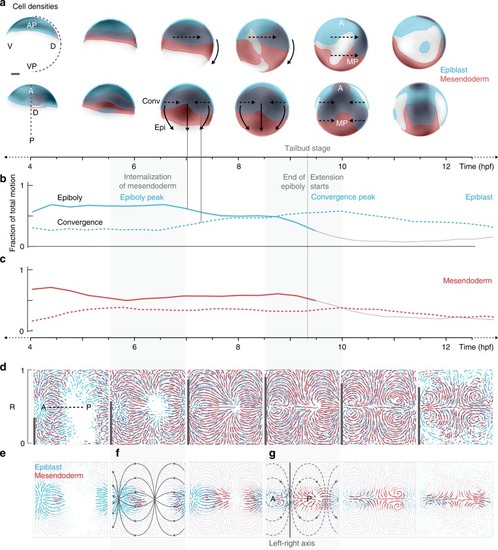
- Title
-
Multi-scale imaging and analysis identify pan-embryo cell dynamics of germlayer formation in zebrafish
- Authors
- Shah, G., Thierbach, K., Schmid, B., Waschke, J., Reade, A., Hlawitschka, M., Roeder, I., Scherf, N., Huisken, J.
- Source
- Full text @ Nat. Commun.
|
|
|
|
|
|
|
|
|
|