- Title
-
Integrated K+ channel and K+Cl- cotransporter function are required for the coordination of size and proportion during development
- Authors
- Lanni, J.S., Peal, D., Ekstrom, L., Chen, H., Stanclift, C., Bowen, M.E., Mercado, A., Gamba, G., Kahle, K.T., Harris, M.P.
- Source
- Full text @ Dev. Biol.
|
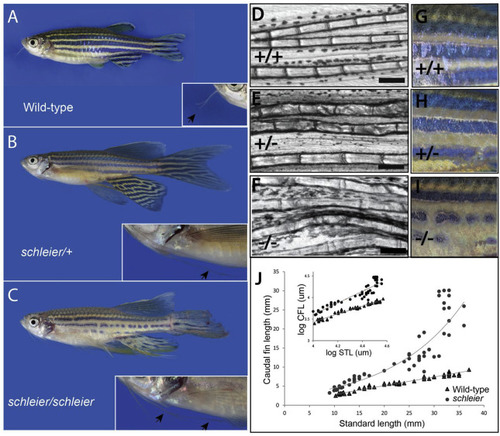
Dominant enhancer of longfin causes increased fin proportion through enhanced relative growth of the fin during development. A, B, C) Phenotype of schleier mutant zebrafish affects fin and barbel proportion and pigmentation of the adult zebrafish in a dose dependent manner. D, E, F) Segment length of lepidotrichia from adult caudal fins of wild-type, heterozygous, and homozygous schleier fish. Bar represents 200 μM. G, H, I) Close up of pigment pattern on flank of wild-type, heterozygous, and homozygous schleier fish. J) Growth curves of caudal fin length in wild-type and schleier heterozygous fish as a function of standard length (STL). Inset, Log-log plot of caudal fin length vs. STL to illustrate allometric scaling of growth. Wild-type: y = 0.9209x - 0.2495, R2 = 0.97. Schleier, y = 1.3498x - 1.7933, R2 = 0.89. PHENOTYPE:
|
|
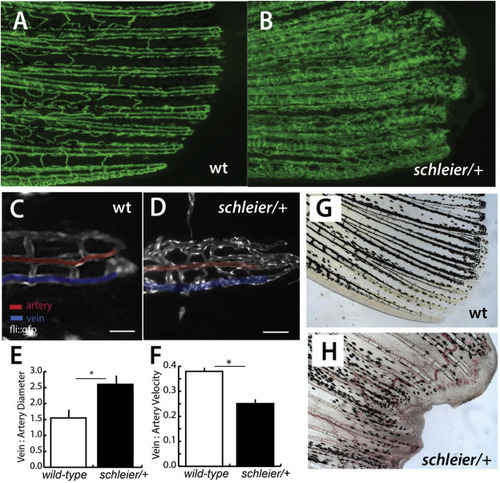
Schleier mutation highlights role of vascular tone associated with altered proportionality in zebrafish fins. A, B) Pattern of vascularization in caudal fins of adult wild-type (wt) and schleier heterozygous fish visualized by Tg(fli1:egfp) transgenic expression of endothelial cells. C, D) Close up of caudal fin tip showing venous and arterial pattern variation in the schleier mutant associated with increased number of endothelial cells. Veins and arteries pseudo-colored blue and red, respectively; scale bar equals 100 μM. E) Measure of vascular dilation in schleier caudal fin tips. Mean ± standard error of mean (SEM) is shown. *, p < 0.05 by ANOVA. N = 3 fish per genotype; mean of 5 artery and 5 vein diameter measurements per fish. F) Quantitation of vascular flow in fin tip in arteries compared with veins. Mean ± standard error of mean (SEM) is shown *, p < 0.05 by ANOVA. N = 3 fish per genotype; mean of 10 arterial red blood cells and 10 venous red blood cells per fish. G, H) Excessive vascularization and blood pooling in the schleier mutant fin tip (H) compared with wild-type fin tip (G). |
|
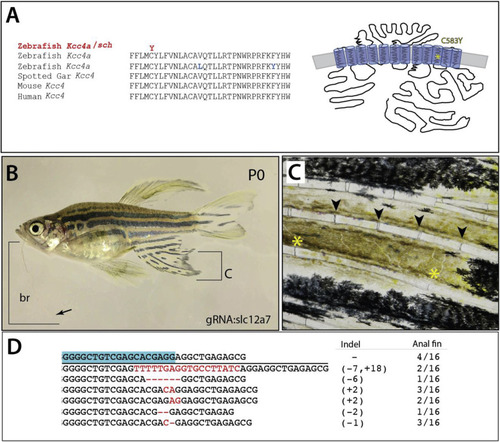
Schleier is caused by mutation of slc12a7a encoding potassium chloride cotransporter Kcc4a. A) Multispecies alignment of predicted coding sequence of slc12a7a showing position of altered residue in Kcc4a in the schleier mutant. Schematic representation redrawn after (Marcoux et al., 2017). B) Adult wildtype P0 zebrafish with somatic clones harboring deletions of slc12a7a/kcc4a. Arrow points to tip of barbel; bracket shows extent of growth. C) Close up of anal fin labeled in (B) showing elongated segments of the rays (joints marked *); normal segmentation pattern observed just above (arrowheads). D) Sequence of deletions identified in affected region of anal fin and frequency (12/16); blue box, sequence of the guide RNA used in targeting. |
|
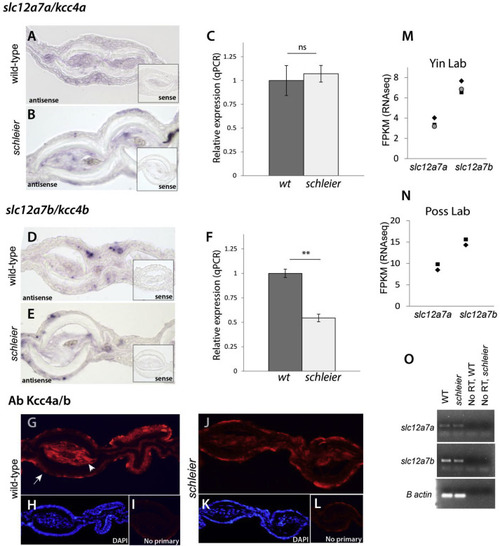
Expression of slc12a7a/kcc4a and its paralog slc12a7b/kcc4b in fin mesenchyme. A, B) In situ hybridization of slc12a7a/kcc4a in cross sections of adult caudal fins of wild-type (A) and heterozygous schleier mutants (B). Inset, signal from control in situ probes on serial sections. C) Quantitative PCR measurement of slc12a7a/kcc4a transcript in wild-type and heterozygous schleier caudal fin tissue. Gene expression was normalized to the geometric mean of three reference genes; n = 4 samples per genotype; 4 adult fins per sample. Error bars indicate SEM; ns, not significant using Student’s t-test. C, D) In situ hybridization of the paralogue slc12a7b/kcc4b in cross sections of adult caudal fins of wild-type (D) and heterozygous schleier mutants (E). Inset, signal from control in situ probes on serial sections. F) Quantitative PCR measurement of slc12a7b/kcc4b transcript in wild-type and heterozygous schleier caudal fin tissue. Gene expression was normalized to the geometric mean of three reference genes; n = 4 samples per genotype; 4 adult fins per sample. Error bars indicate SEM; *, p < 0.01 using Student’s t-test. G-L), Immunofluorescence of antibodies against Kcc4 in adult caudal fins. G, J) Experimental (primary plus secondary antibody) on wild-type and heterozygous schleier fin sections. Arrow, fin epidermis; arrowhead, fin mesenchyme. H, K), DAPI counterstaining of wild-type and heterozygous schleier fin sections. I, L) Signal from control sections hybridized with secondary antibody only. M, N) Number of slc12a7a/kcc4a and slc12a7b/kcc4b transcripts in wild-type caudal fin, measured in fragments per kilobase of transcript per million mapped reads (FPKM). Data were obtained from www.zfregeneration.org (Nieto-Arellano and Sanchez-Iranzo, 2019). O) Endpoint RT-PCR on cDNA synthesized from wild-type (WT) and schleier heterozygous caudal fin RNA. No RT, no reverse transcriptase control. |
|
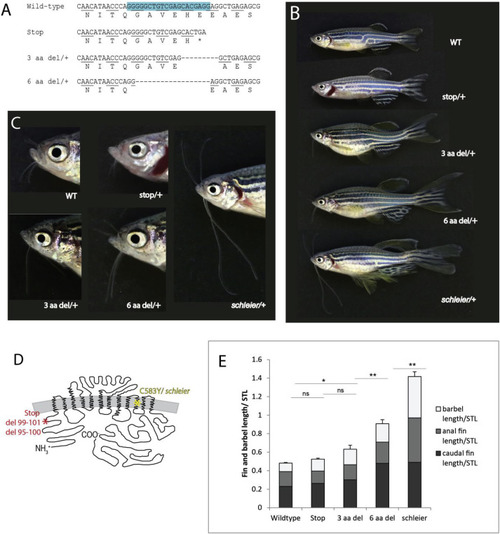
Allelic titration of slc12a7a/kcc4a expressivity revealing necessity and sensitivity to levels of Kcc4a during development. A) Identified deletions in F1 progeny and predicted protein alteration; blue box, sequence targeted by guide RNA. B) Representative pictures of individuals heterozygous for different slc12a7a/kcc4a alleles. C) Barbel phenotypes conferred by different slc12a7a/kcc4a alleles. D) Schematic of Kcc4a protein depicting locations of premature stop codon, 3 amino acid deletion, 6 amino acid deletion (red asterisk), and schleier mutation (yellow asterisk). E) Tabulation of relative length of fins and barbels in F1 progeny heterozygous for each genotype; mean ± SEM is shown. n.s., not significant; *p < 0.05; **p < 0.01 calculated using a one-way ANOVA followed by a Tukey’s multiple comparisons test; n = 6–19 fish per genotype. PHENOTYPE:
|
|
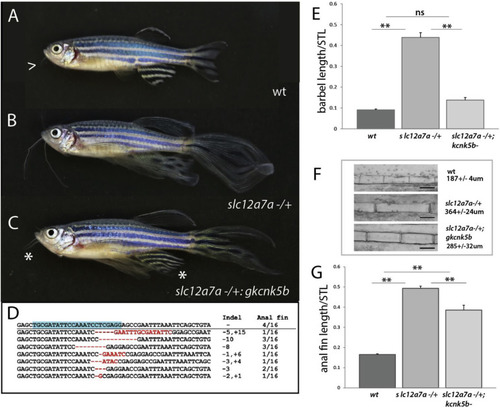
Regulation of size in schleier requires functional kcnk5b. A-C) Representative pictures of growth phenotypes of zebrafish with altered potassium channel function. A) Wild-type adult zebrafish; arrowhead, end of barbel. B) schleier heterozygote. C) schleier heterozygote fish harboring somatic deletion clones within kcnk5b, (gkcnk5b); asterisk demarcates reduction of overgrowth phenotype of the barbel and anal fins. D) Deletions detected in kcnk5b from reverted anal fins in affected mutants. Blue highlight = targeted sequence. E-G) Reversion of schleier overgrowth phenotypes after abrogation of kcnk5b function. E) Barbel length relative to standard length (STL). F) Size of segments within anal fin rays. G) Anal fin length relative to STL. For E-G, mean ± SEM is shown for each panel. n.s., not significant; **p < 0.01 calculated using a one-way ANOVA with a Tukey’s multiple comparisons test; n = 7–24 fish per genotype. |
Reprinted from Developmental Biology, 456(2), Lanni, J.S., Peal, D., Ekstrom, L., Chen, H., Stanclift, C., Bowen, M.E., Mercado, A., Gamba, G., Kahle, K.T., Harris, M.P., Integrated K+ channel and K+Cl- cotransporter function are required for the coordination of size and proportion during development, 164-178, Copyright (2019) with permission from Elsevier. Full text @ Dev. Biol.