- Title
-
Live fate-mapping of joint-associated fibroblasts visualizes expansion of cell contributions during zebrafish fin regeneration
- Authors
- Tornini, V.A., Thompson, J.D., Allen, R.L., Poss, K.D.
- Source
- Full text @ Development
|
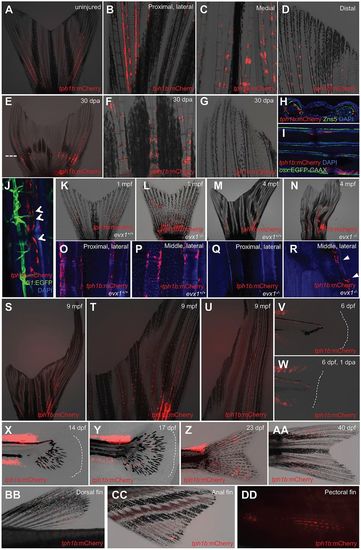
tph1b+ fibroblasts localize to adult zebrafish fin joints. (A-D) tph1b:mCherry expression in uninjured adult zebrafish caudal fins. (A-D) Whole fin (A), and magnified proximal dorsal rays (B), medial rays (C) and distal mediolateral rays (D). tph1b:mCherry is expressed near fin joints. (E-G) tph1b:mCherry expression in whole (E), medial (F) or distal (G) caudal fins at 30 days post-amputation (dpa). Dashed line indicates the amputation plane. (H) Transverse section of uninjured tph1b:mCherry rays, co-stained with DsRed (red, tph1b:mCherry), ZNS-5 (osteoblasts, green) and DAPI (nuclei, blue). (I,J) Optical section through uninjured tph1b:mCherry; osx:EGFP-CAAX (I) or tph1b:mCherry; fli1:EGFP (J) fin ray, stained with DAPI (nuclei, blue). tph1b:mCherry-expressing cells do not colocalize with osteoblast markers or fli1:EGFP-expressing cells (arrowheads, J). (K-N) Wholemounted tph1b:mCherry caudal fins in wild-type (K,M) or jointless evx1−/− (L,N) background at 1 (K,L) or 4 (M,N) months post-fertilization (mpf). (O-R) Optical sections of tph1b:mCherry fin rays in a wild-type (O,P) or evx1−/− (Q,R) background, showing proximal (O,Q) or middle (P,R) lateral rays stained with DAPI (nuclei, blue). tph1b:mCherry localizes to proximal lateral rays and middle lateral rays near mature joints, but is absent in jointless fins, except for injury-induced expression (R, arrowheads). (S-U) tph1b:mCherry expression in the longfin (lof) background, shown at 9 mpf in lateral (T) and distal (U) ray tissues. (V,W) tph1b:mCherry expression at 6 dpf (V) or at 6 dpf, 1 dpa (W). Expression is not induced in larval finfold tissue after injury. Dashed lines indicate the tailfin boundary. (X-AA) tph1b:mCherry expression during caudal fin development at 14 (X), 17 (Y), 23 (Z) and 40 (AA) dpf. Dashed lines indicate fin boundary. (BB-DD) tph1b:mCherry expression in adult dorsal (BB), anal (CC) and pectoral (DD) fins, excluding distal segments. EXPRESSION / LABELING:
PHENOTYPE:
|
|
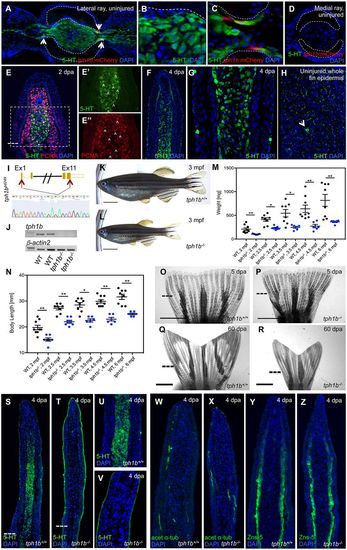
Tph1b is dispensable for fin regeneration but important for organismal growth. (A-D) Transverse section of lateral (A-C) or medial (D) rays of uninjured tph1b:mCherry caudal fins, stained for serotonin (5-HT, green) and nuclei (DAPI, blue). (B) Intracellular serotonin expression in fibroblasts. (C) Hemiray region shows tph1b+ cells (arrows) occasionally colocalized with serotonin staining. (D) Weak tph1b:mCherry expression, and no serotonin expression, in medial rays. Dashed lines outline the hemiray. (E) Longitudinal section of a 2 dpa blastema, stained for serotonin (5-HT, green), proliferating cells (PCNA, red) and nuclei (DAPI, blue). Serotonin is present in blastemal progenitors. Dashed line indicates the amputation plane. Separate channels for 5-HT (E′, green) and PCNA (E″, red) in the boxed area in E. Arrowheads indicate cells co-labeling for 5-HT and PCNA. Dashed line indicates the amputation plane. (F,G) Longitudinal sections of a 4 dpa blastema, stained for serotonin (5-HT, green) and nuclei (DAPI, blue). (G) Intracellular serotonin synthesis in fibroblasts and vesicular serotonin in some superficial epidermal cells. (F) 5-HT staining was occasionally detected in distal basal epithelial cells. (H) Optical section of uninjured whole caudal fin epidermis, stained for serotonin (5-HT, green) and nuclei (DAPI, blue). Stronger expression (arrow) in putative gland cells. (I) Schematic of the tph1b gene sequence, sgRNA target sites (red arrows, top) and chromatogram verification of tph1b sequence deletion (bottom). (J) RT-PCR for tph1b or β-actin2 on 3 dpa fins from tph1b−/− or wild-type siblings. tph1b mRNA (exons 6-7) is absent in tph1b−/− mutant fins. n=3 for each group. (K,L) Three mpf wild-type (J) or tph1b−/− (K) zebrafish. tph1b−/− fish are significantly smaller than wild-type siblings. At least four separate clutches were analyzed. (M,N) Mass (mg) (M) or body length (mm) (N) of wild type (black) or tph1b mutants (tph1b−/−, blue) (data are mean±s.e.m.). *P<0.01, **P<0.001; Mann–Whitney two-tailed test, n=6-9 per group. (O-R) Bright-field images of fins at 5 (O,P) or 60 dpa (Q,R). Wild-type (O,Q) and tph1b−/− (P,R) fins regenerate normally, despite differences in body size. Dashed lines indicate the amputation plane. (S-V) Longitudinal sections of wild-type (S) or tph1b−/− (T) fins at 4 dpa, stained for serotonin (5-HT, green) and nuclei (DAPI, blue). Higher magnification views are shown in U (tph1b+/+) and V (tph1b−/−). Serotonin synthesis is abrogated in tph1b−/− regenerating fins; epidermal 5-HT is still detected. Dashed lines indicate the amputation plane. (W-Z) Longitudinal sections of wild-type (V,Y) or tph1b−/− (W,Z) fin rays at 4 dpa, stained for osteoblasts (ZNS-5, green) (W,X) or nerves (acetylated α-tubulin, green) (Y,Z) and nuclei (DAPI, blue). tph1b−/− fins display normal osteoblast and nerve marker expression. Scale bars: 5 mm in K,L; 2 mm in O-R. EXPRESSION / LABELING:
PHENOTYPE:
|
|
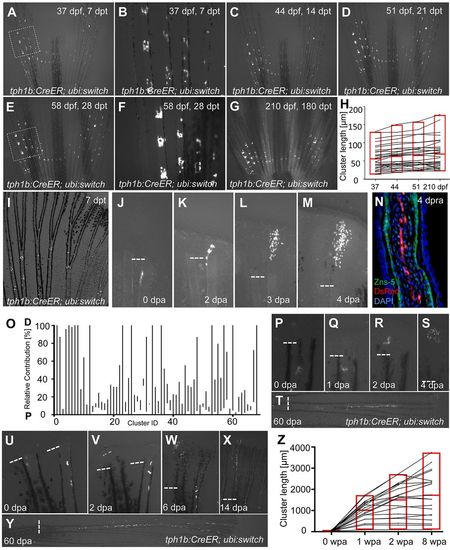
tph1b+ fibroblasts contribute to peri-joint regions in the absence of injury but broaden contributions during regeneration. (A-G) tph1b:CreER; ubi:switch fish were treated with 4 µM tamoxifen at 30 dpf, and labeled cells in caudal fins were tracked at 7 (A,B), 14 (C), 21 (D), 28 (E,F) and 180 (G) days post-tamoxifen treatment (dpt). The boxed areas in A and E are magnified in B and F, respectively, showing limited contributions of tph1b+ cells and progeny to local joint tissue throughout adulthood. (H) Plot of tph1b+ proximodistal (PD) cluster lengths (in µm) during normal growth measured at 37, 44, 51 and 210 dpf. tph1b:CreER; ubi:switch fish were treated as in A. n=31 clusters. Labeled cells remained closely associated to joints. Red box indicates minimum/maximum values, with the average value in the middle. (I) An uninjured adult tph1b:CreER; ubi:switch caudal fin, treated with 4 µM tamoxifen and imaged at 7 dpt. tph1b+ cells near joints are labeled. (J-M) Contribution of a labeled tph1b+ cell in tph1b:CreER; ubi:switch fish, shown at 0 (J), 2 (K), 3 (L) and 4 (M) dpa. Dashed line indicates amputation plane. (N) Longitudinal section of a tph1b:CreER; ubi:switch fin regenerate at 4 days post-re-amputation (dpra), stained for osteoblasts (ZNS-5, green), mCherry (DsRed, red) and nuclei (DAPI, blue). We saw no evidence for contributions by tph1b+ joint-associated cells to osteoblasts during regeneration. (O) Plot of final relative position along the PD axis in which tph1b+ cell progeny are distributed, normalized for regenerated ray length as a percentage (0, proximal; 100, distal). Fins were amputated near labeled tph1b+ cells and progeny were tracked through 60 dpa. n=68. P, proximal; D, distal. (P-T) Contribution of a tph1b+ labeled cell in tph1b:CreER; ubi:switch fish, shown at 0 (P), 1 (Q), 2 (R), 4 (S) and 60 (T) dpa. Joint-associated tph1b+ cells can contribute progeny to regenerated tissue well beyond joint areas. (U-Y) Contribution of a tph1b+ labeled cell in tph1b:CreER; ubi:switch fish (left ray), shown at 0 (U), 2 (V), 6 (W), 14 (X) and 60 (Y) dpa. Joint-associated tph1b+ cells can contribute progeny across the entire PD axis of the regenerate. (Z) Plot of tph1b+ progeny lengths (in µm) during fin regeneration. Adult tph1b:CreER; ubi:switch fish were treated as in I. At 7 dpt, fins were amputated distal to labeled cells, and progeny lengths were measured at 0, 1, 2 and 8 weeks post-amputation (wpa). n=20. Labeled cells significantly expanded their contributions. EXPRESSION / LABELING:
|
|
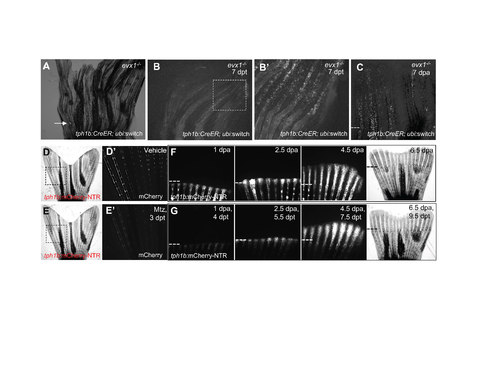
Joint-associated tph1b+ cell progeny are dispensable for fin regeneration. (A) tph1b:CreER; ubi:switch; evx1-/- fish were treated with 4 μM tamoxifen to induce recombination and cell labeling. A few cells in crushed areas (arrow) were labeled. (B) tph1b:CreER; ubi:switch; evx1-/- fish were treated with 4 μM tamoxifen to induce recombination and cell labeling. Rays with more severe injuries show labeled tph1b+ cells at 7 days post-treatment (dpt). Box in (B) magnified in (B’). (C) tph1b:CreER; ubi:switch; evx1-/- fish were treated with 4 μM tamoxifen, and rays with labeled cells were amputated at 7 dpt and tracked through 7 dpa. Injury-induced tph1b+ cells can contribute to regenerating fin tissue. Dashed line, amputation plane. (D, E) tph1b:mCherry-NTR fish were treated with vehicle (D) or metronidazole (Mtz) (E). mCherry signal for boxes in (D) and (E) are shown in (D’) and (E’) at 3 days posttreatment (dpt). (F, G) Fins of vehicle (F)- or Mtz (G) -treated tph1b:mCherry-NTR fins were amputated and imaged at at 1, 2.5, 4.5, or 6.5 dpa. No gross differences in fin regeneration were observed following tph1b+ cell ablation. n = 6 for vehicle-treated; n = 4 for Mtz-treated. Dashes lines, amputation plane. EXPRESSION / LABELING:
|