- Title
-
Neurotrophic effects of progranulin in vivo in reversing motor neuron defects caused by over or under expression of TDP-43 or FUS
- Authors
- Chitramuthu, B.P., Kay, D.G., Bateman, A., Bennett, H.P.
- Source
- Full text @ PLoS One
|
Inhibition of zfPGRN-A translation resulted in the generation of shorter axons and stalling of CaP Motor Neurons (MNs) at the horizontal myoseptum. Fig 1A Inhibition of zfPGRN-A translation produced truncated axons in live zebrafish. Compared to control (A1), zfPGRN-A knockdown produced shorter axons (A2) that were rescued by hPGRN mRNA (A3) while hPGRN alone did not produce truncated axons but increased branching (A4). Lateral views (anterior to the left; dorsal to the top) of embryos obtained from the Hb9:GFP transgenic fish. Images were captured at 5X magnification and the hatched box was further subject to 4-5X Zoom. Dashed lines represent the horizontal myoseptum (HM). The double- headed arrows represent increased branching and the white arrow represents axonal length.Fig 1B Expression of the short form zebrafish granulin zfPGRN-1 did not reverse the phenotype due to zfPGRN-A knockdown. B1. zfPGRN-1 did not rescue locomotor defects produced by the knockdown of zfPGRN-A assessed by employing touch evoked swimming test. B2 zfPGRN-1 did not rescue motor axon defects produced by zfPGRN-A Knockdown. The ability of PGRN mRNA to rescue motor neuron defects in zebrafish is therefore limited to some but not all PGRN proteins. MN axonal length from the specified areas in the spinal cord region was measured using imageJ by tracing the labelled MN axons. PHENOTYPE:
|
|
Neuromuscular developmental characteristics of zfPGRN-A knockdown embryos. Fig 2A. Embryos injected with zfPGRN-A AMO displayed reduced overall body length (A1) and a corresponding reduction in the length of the spinal cord (A2). Co-injection of hPGRN mRNA with zfPGRN-A AMO (zfPGRN-A AMO+hPGRN) significantly reversed these phenotypes while hPGRN mRNA alone had no effect on overall body length or the length of the spinal cord (Fig 2A). The spinal cord length or embryo length were measured by tracing spinal cord or embryo using imageJ. Fig 2B zfPGRN-A knockdown resulted in CaP MNss stalling at the horizontal myoseptum as shown by the failure of axonal outgrowth to extend beyond the horizontal myoseptum for up to 72hpf Controls demonstrate normal outgrowth (B1, B2, B3). In the embryos expressing a reduced level of zfPGRN-A CaP MNs are stalled at HM (B4, B5, B6). The panels show lateral views (anterior to the left; dorsal to the top) of embryos obtained from the HB9:GFP transgenic fish in which MNs express HB9 promoter-driven GFP at 30hpf (B1, B4) 48hpf (B2, B5) and 72hpf (B3, B6). Images were captured at 5X magnification and the hatched box was further subjected to 4–5 X Zoom. Dashed lines represent a horizontal myoseptum (HM). Observed phenotypes were normal MN development (Control 30hpf (B1), 48hpf (B2), 72hpf (B3); white arrows) and truncated or shorter axons (zfPGRN-A AMO 30hpf (B4), 48hpf (B5), 72hpf (B6); white arrows). Fig 2C PGRN PGRN deficient embryos displayed aberrant somatic boundaries as well as disorganized and mislocated AChR aggregates in the neuromuscular junctions of the somatic muscles. Panel C1 shows a lateral view of the wild type 27hpf embryo. The boxed area is representative of the areas shown in panels C2 and C3 and dashed lines indicate the horizontal myoseptum. AChR clusters were visualized by alpha-Bungarotoxin staining of control embryos at 27hpf (C2)) and in embryos injected with PGRN-A MO (C3). PGRN-A knockdown embryos show aberrant somite boundaries compared with wild type embryos as indicated by tracing of somite boundaries. PGRN-A knockdown embryos also displayed disorganized and mislocated AchR clusters AChR aggregates compared with wild type embryos. Arrows indicate somite boundaries. Arrowheads denote AChR aggregates. PHENOTYPE:
|
|
PGRN rescues motor axon defect produced by TDP-43 and FUS knockdown or expression of their mutant mRNAs. Lateral views (anterior to the left; dorsal to the top) of embryos obtained from the HB9:GFP transgenic fish. Fig 4A. Compared to controls (A1) embryos injected with TDP-43 AMO produced truncated axons (A2). Embryos co-injected with hPGRN mRNA (TDP-43 AMO+hPGRN) partially reversed the truncation phenotype (A3). Fig 4B. Compared to control (B1), embryos injected with FUS AMO produced truncated axons (B2). Embryos co-injected with hPGRN mRNA (FUS AMO +hPGRN) reversed the truncation phenotype (B3). Fig 4C. Compared to controls (C1), embryos injected with TDP43 (G348C) produced truncated axons (C2). Embryos co-injected with hPGRN mRNA (TDP43 (G348C)+hPGRN) reversed the truncation phenotype (C3). Fig 4D. Compared to controls (D1), embryos injected with FUS (R521H) produced truncated axons (D2). Embryos co-injected with hPGRN mRNA (FUS (R521H)+hPGRN) reversed the truncation phenotype (D3). Dashed line indicates the horizontal myoseptum. Arrow points to a single axon. Images were captured at 5X magnification and the hatched box was further subject to 4-5X Zoom. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|
|
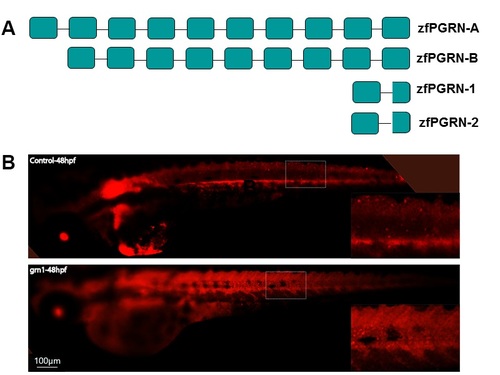
Diagrammatic representation of the mRNA encoding Zebrafish PGRN proteins and validation of PGRN-1 expression with the embryos injected with Grn1 mRNA. Diagrammatic representation of the mRNA encoding Zebrafish PGRN proteins. zebrafish PGRN extend gene family with 4 Grn genes. Grn A and Grn B encodes PGRN protein with 9 and 10 grn motif respectively while Grn-1 and Grn-2 encodes PGRN protein with one and a half grn motif. S1B Fig PGRN-1 is expressed within Grn1 mRNA injected embryos not in control within 48hpf embryos. |
|
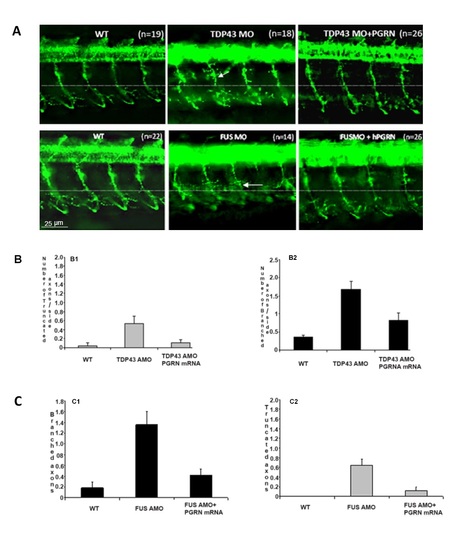
PGRN rescues motor axon defects produced by TDP-43 or FUS knockdown in WT embryos. S2A Top panel. PGRN rescues motor axon defects produced by TDP-43 Knockdown in WT embryos. TDP-43 knockdown produced shorter axons that were rescued by co-injection of hPGRN mRNA. Lateral views (anterior to the left; dorsal to the top) of embryos labelled with znp1 mAb at 27 hpf in wild-type embryos, embryos injected with TDP-43 AMO, embryos co-injected with hPGRN mRNA (TDP-43AMO+hPGRN). Embryos co-injected with hPGRN mRNA (TDP-43AMO+hPGRN) partially reversed the truncation phenotype. Observed phenotypes were normal MN development (WT), increase in truncated and branched axons (TDP43 MO) and partial rescue of truncated MNs (TDP43 MO+PGRN). Dashed lines represent the horizontal myoseptum. S2A Fig bottom panel. PGRN rescues motor axon defects induced by FUS knockdown. FUS knockdown produced shorter axons that were rescued by hPGRN mRNA. Lateral views (anterior to the left; dorsal to the top) of embryos labelled with znp1 mAb at 27 hpf in wild type embryos, embryos injected with FUS AMO, embryos co-injected with hPGRN mRNA (FUS AMO +hPGRN). Embryos co-injected with hPGRN mRNA (FUS AMO+hPGRN) partially reversed truncation phenotype. Observed phenotypes were normal MN development (WT), increase in truncated and branched axons (FUS MO) and partial rescue of truncated MNs (FUS MO+PGRN). Dashed lines represent horizontal myoseptum. Images were captured at 20X magnification and the hatched box was further subject to 4-5X Zoom. S2B Fig TDP-43 knockdown and partial rescue with over-expression of PGRN mRNA in WT embryos. Average number of Truncated (B1) and Branched (B2) CaP MNs per group. S2C Fig. FUS knockdown and partial rescue with over-expression of PGRN. Average number of Branched (C1) and Truncated (C2) CaP MNs per group. |