- Title
-
Myosin phosphatase Fine-tunes Zebrafish Motoneuron Position during Axonogenesis
- Authors
- Bremer, J., Granato, M.
- Source
- Full text @ PLoS Genet.
|
Notochord cell expansion and shift during motor axon outgrowth. (A-D) Time-lapse of a developing zebrafish embryo with transgenic expression of GFP in motoneurons (mnx1:GFP), starting at 22 hpf for 840 min. (A, B) Low magnification bright field images, generated by stitching together several images. (C, D) Higher magnification images of approximately the boxed areas in A (different embryos), in brightfield and GFP, generated by overlaying substacks containing motor neurons and notochord cells, respectively. While the embryo grows and axons are extending, there is a progressive posterior shift of an ‘identified’ notochord cell (outlined by dashed yellow circle) relative to a GFP positive CaP motoneuron (white asterisk). (E) Schematics of this shift. Note that as CaP motor axons (green) are extending and notochord cells (yellow dotted circle) shift posteriorly, the diameter of notochord cells increases, quantified in (F; n = 9). (G) Velocity of posterior shift of notochord cells relative to motor axons in μm/h over time (n = 6). Shift velocity is initially low and peaks between 210 and 630 min. (H-L) Time-lapse imaging of a CaP motoneuron labeled in red (mnx1:mKate) in Evx1:Gal4; UAS:GFP double transgenic embryos from 21 hpf until axons have fully extended to the ventral myotome (620 minutes). Note that an adjacent, GFP positive interneuron (+) and adjacent individual muscle fibers (white brackets) both stay aligned with the motoneuron. In contrast, individually labeled notochord cells (white arrow) shift progressively posteriorly compared to the labeled CaP motoneurons (n = 8/8). See also S1 Movie. EXPRESSION / LABELING:
|
|
A non-sense mutation in mypt1 causes axon guidance errors. (A) Schematics outlining the domains of the wildtype (top) and the truncated mypt1p82emcf protein (bottom). (B) Phosphorylation-dependent regulation of myosin II: Myosin light chain kinase (MLCK) and Rho kinase (ROCK) increase myosin II phosphorylation and thereby enhance myosin II contractility. Conversely, Myosin phosphatase composed of Mypt1, catalytic and regulatory subunits decreases myosin II phosphorylation and thus causes myosin II relaxation. (C) Injection of 250pg wildtype mypt1 mRNA into one-cell stage wildtype (blue bars) and p82emcf mutant embryos (red bars) significantly reduced motor axon guidance errors in mypt1 mutant embryos as assayed at 26 hpf using SV2 staining (p<0.001; two-tailed t-test). (D) In 25 hpf embryos mypt1 mRNA is readily detectable in the spinal cord (dotted line) and in the myotomes (arrowheads). (E) 3D projection image of a cross section through the trunk of a 26 hpf embryo reveals p-MLC expression in slow-twitch muscle cells (green, arrow head) and in fast-twitch muscle fibers (arrow), while in the spinal cord (dashed circle) p-MLC expression levels are below detection limit. Motor axons are stained with SV2 (in red). (F-H) Co-staining of MHC and p-MLC in siblings (F) and mypt1 mutants (G). Compared to MHC levels (F, G), p-MLC levels are increased in mypt1 mutants (G’, G”) when compared to wildtype (F’, F”; quantified in H). (I-K) FRET analysis using the SECFP donor (I pre, I’ post bleaching) and the YPet acceptor (J pre, J’ post bleaching) reveals increased MLC phosphorylation, quantified in K. Note that non-bleached areas (outside of the region of interest, ROI) remained unchanged. |
|
Mypt1 is required in the environment to restrict excessive axon branching. (A, B) SV2 antibody labeling at 25 hpf reveals disorganized motor axon morphologies in mypt1p82emcf mutants (B) compared to wildtype siblings (A). Unlike wildtype CaP motoneurons (C), individually labeled mutant motor axons (using mnx1:mKate) project with frequent changes in projection direction (dots), and exhibit excessive branching (arrowheads). The gray dashed line marks the border of the spinal cord. (E, F) Transplantation of rhodamine-dextran labeled wildtype blastula stage cells into mypt1 mutants analyzed at 26–27 hpf exhibit mutant motor axon phenotypes (E). Conversely, transplantation of mutant motoneurons into a wildtype host results in wildtype-like motor axon projections (F). |
|
mypt1 is required cell-autonomously for motoneuronal positioning. (A-C) SV2 staining (all axons, green) combined with stochastically labeled CaP motor neurons using mnx1:mKate (single cell labeling, red) to determine relative CaP soma positions in 26 hpf wildtype (A) and mypt1 mutant embryos (B). Red arrowheads indicate the position of CaP cell bodies, black arrowheads the position of the axonal exit point. In contrast to wildtype, 33% of mutant CaP cell bodies were shifted rostrally (p = 0.0177, Fisher exact; for detail on quantification, see Material and Methods). Bar graph of relative neuronal position in wildtype and mypt1 mutants (C). Following transplantation, wildtype-derived CaP motoneurons (labeled with rhodamine-dextran, red) exhibited normal motoneuron positioning in 26–27 hpf wildtype embryos (D). In contrast, mypt1 mutant derived CaP motoneuron when transplated into wildtype embryos frequently failed to adjust their position (E; p = 0.0087, Fisher exact). (F-I) Time-lapse analysis of motoneuron membrane dynamics in 18–19 hpf transgenic mnx1:mKate-mnx1:mCD8-mKate embryos, expressing mKate in the cytoplasm and on cell membranes of motoneurons. Motoneurons in mypt1 mutants displayed membrane blebbing (yellow arrowheads in G, G', G"; n = 5; p = 0.0001, Fisher exact), not observed in wildtype siblings (n = 19, F, F', F"). Treatment with the myosin II inhibitor blebbistatin but not with DMSO (H, H', H") completely abolished membrane blebbing in mypt1 mutants (I, I', I"; n = 8,). (J, K) SV2 staining (all axons, green) combined with stochastically labeled CaP motor neurons using mnx1:mKate (single cell labeling, red) to determine relative CaP soma positions in 26 hpf mypt1 mutant embryos treated with DMSO for control (J) or blebbistatin (K). Blebbistatin treatment significantly reduced rostral mispositioning of mypt1 mutant CaP motoneurons (p = 0.0077, Fisher exact). (L-N) N-Cadherin (NCadh, red) staining of fixed embryos carrying the mnx1:mCD8-GFP transgene (green) which labels motoneuronal membranes. Single plane of confocal images with pseudocolored colocalizing pixels generated by Imaris software in a sibling (L) and a mypt1 mutant (M). Percentage of the green volume (mCD8+) which is colocalized is determined to quantify the fraction of NCadh+ cell membrane (N). EXPRESSION / LABELING:
PHENOTYPE:
|
|
Mypt1 maintains motor neuron position. At the onset of axonogenesis at ~19–20 hp CaP motoneurons in wildtype (A) and mypt1 mutant (B) embryos carrying the mnx1:mKate mnx1:mCD8-mKate transgene are located at the points where motor axons exit from the spinal cord (n = 26/27 CaP in wildtype, and n = 12/12 CaP in mypt1 mutants; arrowheads point to the nascent axons). Time-lapse imaging of CaP motoneurons from the onset of axon initiation at 19 hpf until the axons reached the horizontal myoseptum in mnx1:mCD8-GFP transgenic wildtype (C1–5) and mypt1 mutant embryos (D1–5). During the length of the movie (400 min) all wildtype (n = 10/10) and all mutant (n = 25/25) motoneurons retained their position above the spinal cord exit point. Time-lapse imaging of CaP motoneurons labeled with mnx1:mKate in mnx1:mCD8-GFP transgenic siblings (E' to E‴) and mypt1 mutant embryos (F' to F‴) after axons have reached the horizontal myoseptum at 21 hpf until axons have reached the ventral extent of the myotome. While 30/31 wildtype CaP motoneuron cell bodies (red arrowhead) stayed precisely above the exit point (white arrow), 32% (n = 7/22) mypt1 mutant motoneuron cell bodies shifted progressively rostrally (p = 0.006, Fisher exact). A stalling mypt1 mutant axon is seen in an adjacent hemisegment (white star in F). EXPRESSION / LABELING:
|
|
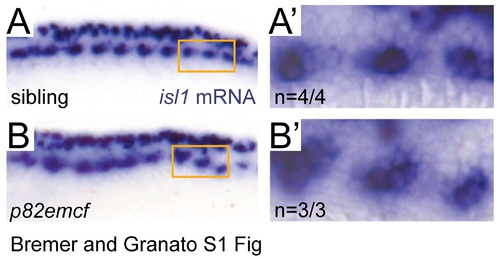
Mypt1 is dispensable for motoneuron specification. (A, B) In situ hybridization for isl-1 in 25 hpf embryos. Rohon Beard neurons in the dorsal spinal cord and motoneurons in the ventral spinal cord both express isl-1 in both, siblings (A) and p82emcf mutants (B). Higher magnification images of the orange-boxed area in the ventral spinal cord containing motoneurons on the right side: sibling (A') and p82emcf mutant (B'), demonstrating normal neuronal specification. |
|
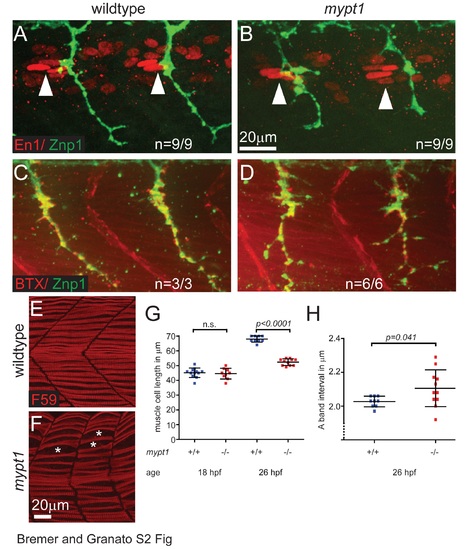
Mypt1 is dispensable for polarity and postsynaptic differentiation of adaxial /slow twitch muscle cells but is required for muscle cell growth. (A, B) Immunostaining for Engrailed-1 (En1, red) and axonal Znp1 (green) at 26 hpf in wildtype (A) and mypt1 mutant embryos (B), showing normal localization of En1 positive elongated nuclei of adaxial muscle cells in the anterior somites (anterior of the motor axons, arrowheads). This indicates normal specification and polarity of adaxial muscle cells in mypt1 mutant embryos. (C, D) Staining with bungarotoxin (BTX, red) and for axonal Znp1 (green) at 26 hpf in wildtype (C) and mypt1 mutant embryos (D), showing normal sites of postsynaptic differentiation in muscle cells directly opposing motor axons. This indicates normal muscle fiber differentiation in mypt1 mutant embryos. (E-H) Immunostaining for myosin heavy chain in adaxial muscle cells (F59, red) at 26 hpf in wildtype (E) and mypt1 mutant embryos (F), showing irregular spacing of muscle cells (stars) and shorter muscle cells in mypt1 mutant embryos. Quantification of muscle fiber length at 18 hpf and 26 hpf (G) showing that mypt1 mutant muscle cells have normal length initially, but fail to grow over time. Quantification of sarcomere length at 26 hpf (H) as determined by the interval of myosin heavy chain rich A-bands, showing that the reduced muscle cell length is not caused by sarcomere shortening, but rather by reduced addition of new sarcomeres. |