- Title
-
Iterative use of nuclear receptor Nr5a2 regulates multiple stages of liver and pancreas development
- Authors
- Nissim, S., Weeksb, O., Talbot, J.C., Hedgepeth, J.W., Wucherpfennig, J., Schatzman-Bone, S., Swinburne, I., Cortes, M., Alexa, K., Megason, S., North, T.E., Amacher, S.L., Goessling, W.
- Source
- Full text @ Dev. Biol.
|
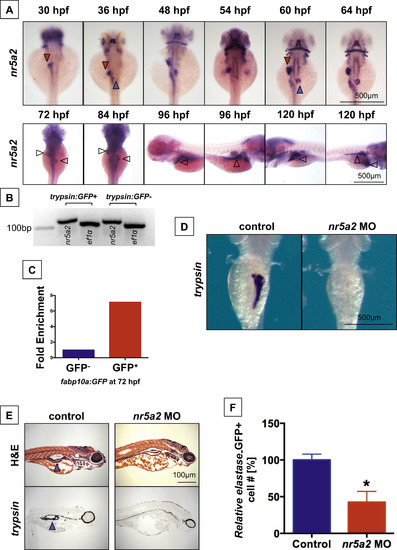
nr5a2 is expressed in the developing endoderm and is required for exocrine pancreas formation. (A) Expression pattern of nr5a2 in the developing endoderm by in situ hybridization. nr5a2 appears in the developing liver bud (red arrow) by 30 hpf and the pancreas bud (blue arrow) by 36 hpf. After 84 hpf, it is expressed in the mature liver (red arrow) and exocrine pancreas (blue arrow). (B) Expression of nr5a2 in FACS isolated acinar cells from the Tg(trypsin:GFP) line at 72 hpf by qRT-PCR. (C) Expression of nr5a2 in FACS isolated hepatocytes from pooled Tg(fabp10a:GFP) embryos at 72 hpf by qRT-PCR (fold enrichment displayed as an average of technical triplicates). (D) Splice-site morpholino (MO) knockdown of Nr5a2 reduces the size of the exocrine pancreas by in situ hybridization for trypsin at 96 hpf. (E) nr5a2 MO injected embryos (96 hpf) have a reduced exocrine pancreas size (blue arrow) by histology and section in situ hybridization for trypsin (n=5). (F) FACS quantification of GFP+cells (% of control) from elastase:GFP reporter fish at 96 hpf demonstrates that MO knockdown of nr5a2 reduces the number of exocrine pancreas cells (n=20 per treatment; p<0.05; unpaired t-test). |
|
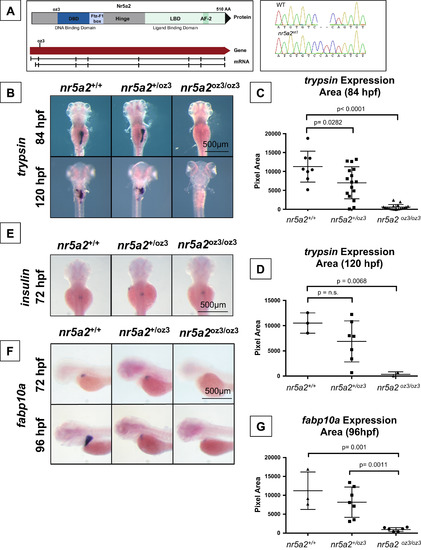
Genetic modulation of Nr5a2 disrupts the formation of the mature exocrine pancreas and liver. (A) The oz3 genetic loss of function mutation is a two base pair insertion within exon 3 of the nr5a2 transcript, before the DNA-binding domain and ligand-binding domain. (B) trypsin in situ hybridization at 84 and 120 hpf reveals that nr5a2oz3/oz3 embryos do not develop a mature exocrine pancreas. (C) Quantification of exocrine pancreas size in nr5a2+/+, nr5a2+/oz3, and nr5a2oz3/oz3 embryos by trypsin area calculations. nr5a2oz3/oz3 and nr5a2+/oz3 embryos have a significantly reduced exocrine pancreas size relative to nr5a2+/+ siblings at 84 hpf (n>8 per group; for nr5a2+/+vs. nr5a2+/oz3 (p=0.0282; for nr5a2+/+vs. nr5a2oz3/oz3, p<0.0001; unpaired t-test). (D) Quantification of exocrine pancreas size in nr5a2+/+, nr5a2+/oz3, and nr5a2oz3/oz3 embryos by trypsin area calculations. At 120 hpf, nr5a2oz3/oz3 have a significantly reduced exocrine pancreas size relative to nr5a2+/+ siblings (n>2 per group; p=0.0068; unpaired t-test). (E) In situ hybridization for insulin demonstrates that nr5a2+/+, nr5a2+/oz3, and nr5a2oz3/oz3 embryos develop an endocrine pancreas. (F) In situ hybridization for fabp10a reveals that nr5a2oz3/oz3 embryos have a reduced liver size at 72 and 96 hpf. (G) Quantification of liver size by fabp10a area calculations demonstrates that nr5a2oz3/oz3 embryos have a significantly reduced liver size relative to nr5a2+/+ and nr5a2+/oz3 categories (n>3 per group; p=0.001; unpaired t-test). EXPRESSION / LABELING:
PHENOTYPE:
|
|
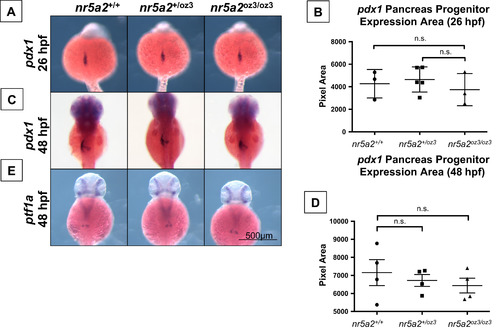
Nr5a2 is not required for the formation of pdx1 and ptf1a pancreas progenitors. (A) The area of pdx1 pancreas progenitors is similar across nr5a2+/+, nr5a2+/oz3, and nr5a2oz3/oz3 embryos at 26 hpf. (B) No significant differences in pdx1 progenitor population size identified by a quantification of pdx1 expression area by genotype (p>3 per group; for nr5a2+/+vs. nr5a2+/oz3, p=0.6746; for nr5a2+/+vs. nr5a2oz3/oz3, p=0.6606; unpaired t-test). (C) pdx1 pancreas progenitors are formed at 48 hpf in nr5a2+/oz3 and nr5a2oz3/oz3 embryos; however, subtle difference in pdx1 progenitor budding is noted with variable penetrance across the nr5a2oz3/oz3 population. (D) No significant differences in the size of the pdx1 pancreas progenitor pool were observed by quantification of expression area at 48 hpf (n>3 per group; for nr5a2+/+vs. nr5a2+/oz3, p=0.6024; for nr5a2+/+vs. nr5a2oz3/oz3, p=0.4178; unpaired t-test). (E) ptf1a progenitors are formed in nr5a2+/oz3 and nr5a2oz3/oz3 embryos by 48 hpf. |
|
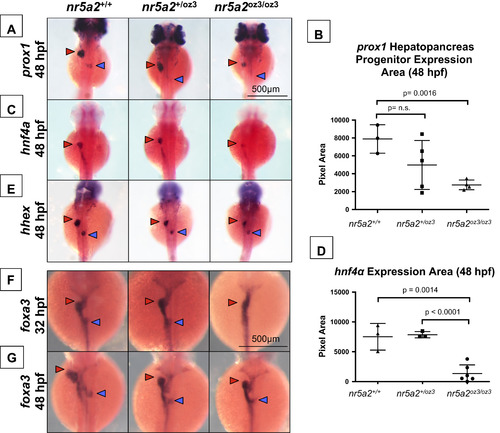
A subset of hepatopancreas progenitors require nr5a2 for specification. (A) nr5a2oz3/oz3 mutants have reduced prox1 expression in the liver (red arrow) and pancreas (blue arrow) buds relative to nr5a2+/+ controls at 48 hpf. (B) Quantification of prox1 expression area in the combined liver and pancreas buds (n>3 per group; for nr5a2+/+ vs. nr5a2+/oz3, p=0.1502; for nr5a2+/+vs. nr5a2+/oz3, p=0.0016; unpaired t-test). (C) In situ hybridization reveals consistent decreased hnf4α expression in the hepatoblasts (red arrow) of nr5a2oz3/oz3 embryos. (D) Quantification of hnf4α expression area in the intestine and liver buds shows significant expression reduction in nr5a2oz3/oz3 embryos relative to nr5a2+/+ and nr5a2+/oz3 groups (n>3 per group; for nr5a2+/+vs. nr5a2oz3/oz3, p=0.0014; for nr5a2+/oz3vs. nr5a2oz3/oz3, p<0.0001; unpaired t-test). (E) The size of the hhex liver bud (red arrow) is reduced in the nr5a2oz3/oz3 embryos compared to nr5a2+/+ siblings, while the pancreas bud (blue arrow) is largely unaffected. (F) In situ hybridization for foxa3 endoderm marker consistently demonstrates delayed gut looping, liver (red arrow), and pancreas (blue arrow) budding in the nr5a2oz3/oz3 embryos at 32 hpf. (G) The sizes of the foxa3 liver (red arrow) and pancreas (blue arrow) buds are consistently reduced in the nr5a2+/oz3 and nr5a2oz3/oz3 mutant populations at 48 hpf. |
|
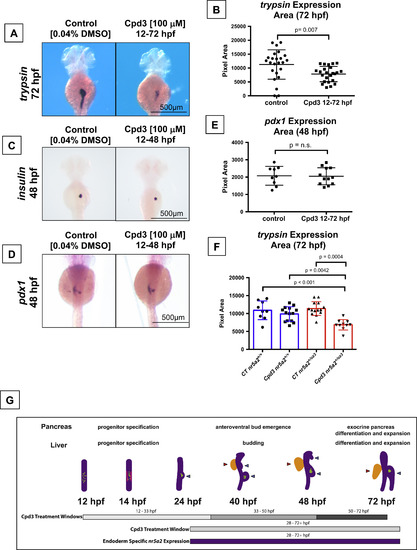
Nr5a2 chemical antagonist Cpd3 impacts exocrine pancreas development in vivo. (A) The Nr5a2 antagonist (Cpd3) produces reduced exocrine pancreas sizes that mimic the nr5a2+/oz3 heterozygotes when treated from 12 to 72 hpf. (B) Quantification of exocrine pancreas size by area of trypsin expression at 72 hpf. Cpd3 significantly reduces exocrine pancreas size compared to vehicle (0.04% DMSO) treated controls (n=24 per group; p=0.007; unpaired t-test). (C) Loss of Nr5a2 activity by Cpd3 treatment does not visibly impact the formation of the insulin expressing β-cells. (D) Cpd3 treatment does not impact the size of the pdx1 pancreas progenitor pool at 48 hpf. (E) Quantification of pdx1 pancreas progenitor expression area at 48 hpf in control and Cpd3 treated groups (n>9 per group; p=0.9089; unpaired t-test). (F) Quantification of exocrine pancreas size by area of trypsin expression at 72 hpf. Heterozygote nr5a2+/oz3 embryos show heightened sensitivity to 100 µM Cpd3 treatment relative to the nr5a2+/+ sibling controls (n>10 per group; for control nr5a2+/+vs. Cpd3 treated nr5a2+/oz3, p<0.001; for control nr5a2+/oz3vs. Cpd3 treated nr5a2+/oz3, p=0.0004; for Cpd3 treated nr5a2+/+vs. Cpd3 treated nr5a2+/oz3, p=0.0042; ordinary one-way ANOVA with Tukey′s multiple comparisons test). (G) Drug treatment windows to examine the impact of Nr5a2 loss of function on distinct stages of pancreas and liver development, including progenitor specification, bud emergence, and organ differentiation and expansion. At 12 hpf, insulin expressing β-cells form within the gut tube (green dots). By 14 hpf, pdx1 expressing pancreas progenitors appear in two stripes (pink lines) An endocrine cell cluster (green) forms (beginning at approximately 24 hpf) and the dorsoventral pancreas bud emerges from the gut tube. By 40 hpf, the dorsoventral and anteroventral buds (blue arrows) have emerged from the gut tube, along with the liver bud (red arrow). Post 50 hpf, the liver and pancreas buds differentiate and expand. Figure adapted (Tiso et al., 2009). EXPRESSION / LABELING:
PHENOTYPE:
|
|
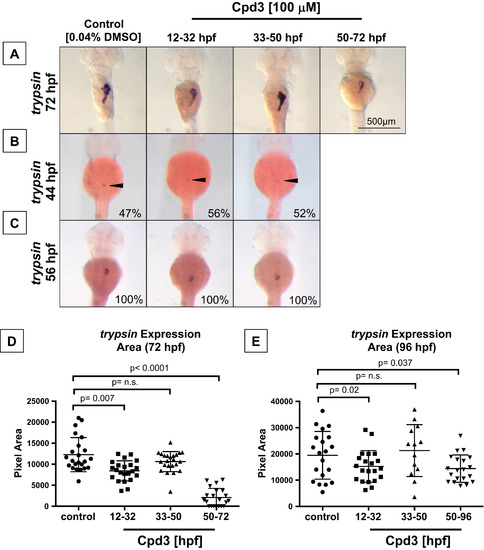
Two temporally defined roles for Nr5a2 during pancreas development. (A) Timed Cpd3 treatment reduces trypsin expression at 72 hpf for the 12-32 hpf and 50-72 hpf treatment windows, but not during the 33-50 hpf window. (B) Control (n=22/46), 12-32 hpf (n=19/36), and 33-50 hpf (n=14/25) treated fish all have the same percentage of fish with differentiated exocrine pancreas cells at 44 hpf, indicating that drug treatments do not alter the timing of initial exocrine cell differentiation. (C) Control (n=12/12), 12-32 hpf (n=11/11), and 33-50 hpf (n=14/14) treated fish all have similarly sized trypsin cell clusters at 56 hpf, demonstrating that initial exocrine pancreas differentiation is unaffected by previous nr5a2 inactivation. (D) Quantification of exocrine pancreas size (by area of trypsin expression at 72 hpf) demonstrates that the 12-32 hpf and 50-96 hpf Cpd3 treatment groups have significantly reduced exocrine pancreas sizes relative to controls. Exocrine pancreas size in the 33-50 hpf treatment group was not significantly altered by treatment (n>21 per group; for control vs. 12-32 hpf, p=0.007; for control vs. 33-50 hpf, p=0.09; for control vs. 50-72 hpf, p<0.0001; unpaired t-test). (E) Quantification of exocrine pancreas size by area of trypsin expression demonstrates that antagonist-induced exocrine pancreas reduction persists through 96 hpf for the 12-32 hpf and 50-96 hpf treatment categories. The size of the pancreas in 33-50 hpf treatment categories remains unchanged relative to the vehicle treated controls (n>13 per group; for control vs. 12-32 hpf, p=0.02; for control vs. 33-50 hpf, p=0.5929; for control vs. 50-72 hpf, p=0.037; unpaired t-test). PHENOTYPE:
|
|
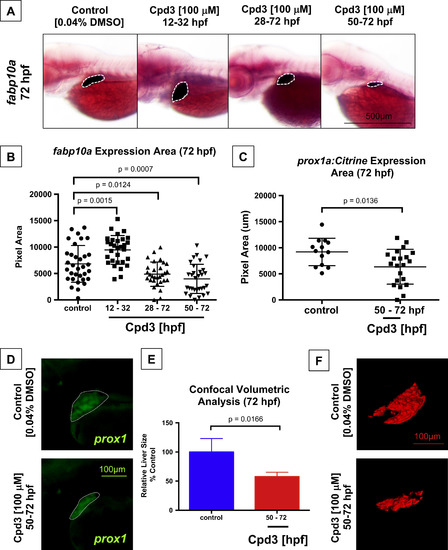
Liver differentiation and outgrowth depends on Nr5a2 activity. (A) Cpd3 treatment reduces the size and alters the position of the fabp10a liver. (B) Quantification of liver size by area of fabp10a expression at 72 hpf. Cpd3 treatment from 12 to 32 hpf enhances the size of the mature liver relative to vehicle (DMSO) treated controls (n>27; p=0.0015; unpaired t-test). Treatment from 28 to 72 hpf and 50-72 hpf reduces the median liver size relative to vehicle (DMSO) treated controls (n>27; p=0.0124; p=0.0007; unpaired t-test). (C) Quantification of liver bud size by area of fluorescence expression in the Tg(prox1a:Citrine) reporter line at 72 hpf. Cpd3 treatment from 50 to 72 hpf significantly reduces the size of the prox1+ liver (n>13; p=0.0136). (D) Fluorescent microscopy images of the embryonic liver in Tg(prox1a:Citrine) reporters following vehicle (DMSO) or Cpd3 treatment from 50 to 72 hpf. (E) Confocal volumetric analysis of liver volume in control (DMSO) and Cpd3 treated (50-72 hpf) Tg(fabp10a:GFP) lines at 72 hpf demonstrates that Cpd3 treatment significantly reduces the volume of the liver (n>3; p=0.0166). (F) Representative 3D renderings of 72 hpf GFP+ livers from the Tg(fabp10a:GFP) line that were exposed to control DMSO or Cpd3 treatment from 50 to 72 hpf. Cpd3 (50-72 hpf) livers are visibly smaller than the control livers. EXPRESSION / LABELING:
PHENOTYPE:
|
|
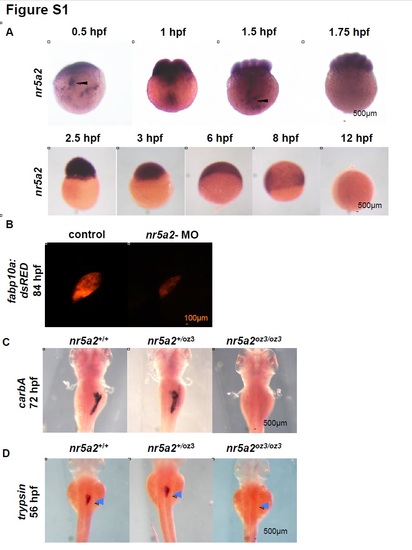
Nr5a2 is required for the formation of the mature pancreas and liver. A) nr5a2 expression in zygote through early segmentation stages. nr5a2 is maternally deposited and expressed in the dividing blastomeres. nr5a2 mRNA is actively shuttled to the dividing blastomeres in vesicles (black arrows) by the 1- cell to 2- cell stage. Expression of nr5a2 persists to approximately 12 hpf, after which it is temporarily inactivated. B) nr5a2 morpholino injected Tg(fabp10a:DsRed) embryos have a reduced embryonic liver at 80 hpf. C) nr5a2oz3/oz3 embryos lack a mature exocrine pancreas by in situ hybridization for carboxypeptidase A5 (carbA). D) trypsin+ acinar cells fail to form in the pancreas region (blue arrows) of nr5a2oz3/oz3 mutant embryos by 56 hpf. EXPRESSION / LABELING:
PHENOTYPE:
|
|
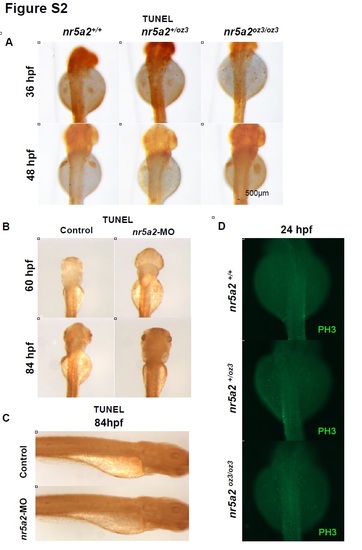
nr5a2 mutants and morphants display normal cell death and proliferation programs. A) TUNEL staining in nr5a2+/+, nr5a2+/oz3, and nr5a2oz3/oz3 mutant embryos at 36 - 48 hpf. nr5a2oz3/oz3 mutants do not show indications of excess cell death relative to nr5a2+/+ sibling controls. B) TUNEL staining in nr5a2 morphant embryos at 60 - 84 hpf. nr5a2 morphants do not show indications of excess cell death relative to controls, demonstrating that cell death is not a likely cause of liver or exocrine pancreas reduction. C) TUNEL staining in nr5a2 morphant embryos at 84 hpf. nr5a2 morphants do not show indications of excess cell death relative to controls, demonstrating that cell death is not a likely cause of exocrine pancreas reduction. D) Immunohistochemistry for phospho-histone 3 (PH3) at 24 hpf. nr5a2oz3/oz3 embryos have no indication of reduced cellular proliferation in the endoderm region. |
|
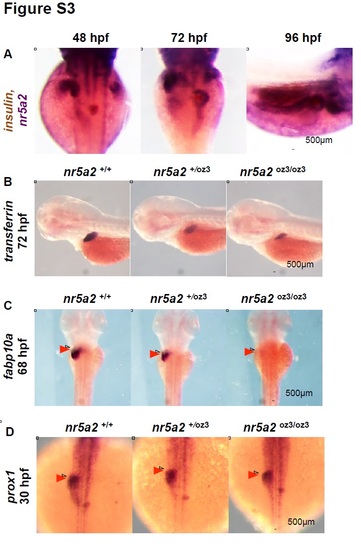
Additional nr5a2oz3 mutant phenotyping A) Double in situ hybridization for insulin (brown) and nr5a2 (purple) from 48 - 96 hpf. nr5a2 and insulin are independently expressed from 72 - 96 hpf. B) In situ hybridization for mature hepatocyte marker transferrin A demonstrates that nr5a2oz3/oz3 embryos have a reduced liver size. C) In situ hybridization for fabp10a at 68 hpf reveals that fabp10a+ hepatocytes fail to form in the liver region of nr5a2oz3/oz3 embryos (red arrows). D) nr5a2oz3/oz3 embryos have normal prox1 expression in the liver and pancreas regions at 30 hpf. |
|
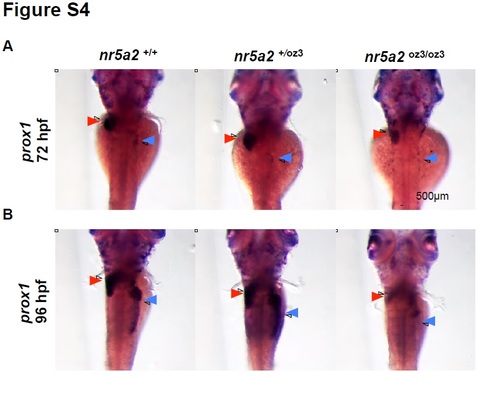
Prox1 expression in the nr5a2oz3 mutant liver and pancreas regions A) prox1 expression is reduced in the liver bud (red arrows) and absent in the pancreas bud (blue arrows) of nr5a2oz3/oz3 embryos at 72 hpf. B) prox1 expression is reduced in the liver bud (red arrows) and absent in the pancreas bud (blue arrows) of nr5a2oz3/oz3 embryos at 96 hpf, demonstrating that prox1 expression in the endoderm does not recover. |
|
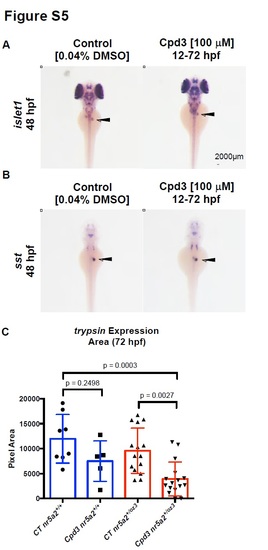
Effects of Cpd3 exposure on pancreas development. A) Endocrine pancreas cells (black arrows) expressing islet1 are present by in situ hybridization in embryos treated with Cpd3 from 12 - 72 hpf. B) δ-cells expressing somatostatin (sst) (black arrows) are present by in situ hybridization in embryos treated with Cpd3 from 12 - 72 hpf. C) Quantification of exocrine pancreas size by area of trypsin expression at 72 hpf. 200 µM Cpd3 treatment elicits a robust reduction in pancreas size in heterozygote nr5a2+/oz3 embryos relative to control nr5a2+/oz3 siblings and control nr5a2+/+ embryos. (n > 5 per group; for control nr5a2+/+ vs. Cpd3 treated nr5a2+/+, p = 0.2498; for control nr5a2+/oz3 vs Cpd3 treated nr5a2+/oz3, p = 0.0027; for control nr5a2+/+ vs. Cpd3 treated nr5a2+/oz3, p = 0.0003; ordinary one-way ANOVA with Tukey’s multiple comparisons test). |
|
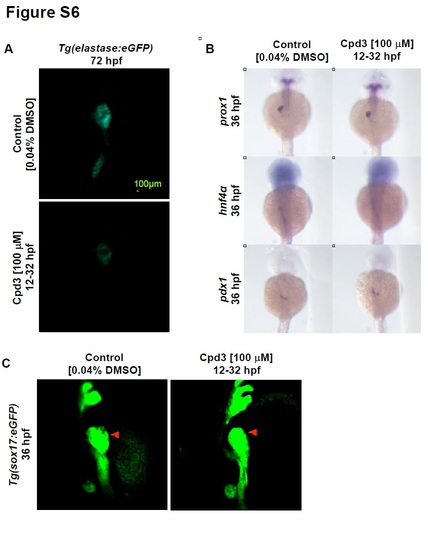
Effects of 12 - 32 hpf Cpd3 exposure on pancreas, liver, and hepatopancreas progenitor development. A) Cpd3 treatment from 12 - 32 hpf reduces the size of the exocrine pancreas in Tg(elastase:eGFP) embryos at 72 hpf. B) Cpd3 treatment from 12 - 32 hpf does not impact the size of the prox1, hnf4α, and pdx1 liver and pancreas progenitor populations. C) Cpd3 treatment from 12 - 32 hpf does not increase the size of the liver bud (red arrow) by confocal imaging of Tg(sox17:eGFP) embryos. |
|
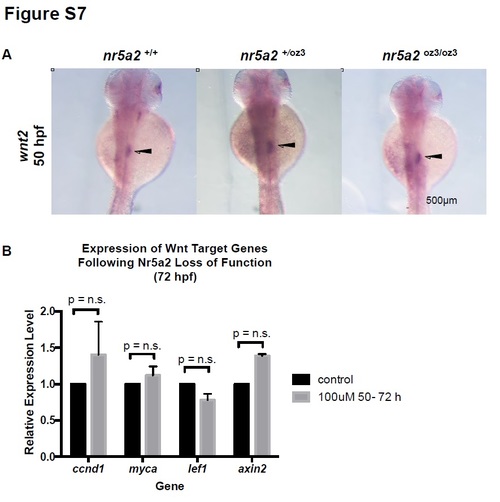
Wnt expression and signaling in Nr5a2 mutants and morphants. A) wnt2 expression in the mesoderm region between the swim bladder and pancreas (arrows) is unaffected in nr5a2+/oz3 and nr5a2oz3/oz3 embryos relative to controls. B) qPCR for Wnt target genes in whole embryos reveals that alterations in Nr5a2 activity by antagonist Cpd3 from 50 - 72 hpf does not significantly impact Wnt pathway activity. |
Reprinted from Developmental Biology, 418(1), Nissim, S., Weeksb, O., Talbot, J.C., Hedgepeth, J.W., Wucherpfennig, J., Schatzman-Bone, S., Swinburne, I., Cortes, M., Alexa, K., Megason, S., North, T.E., Amacher, S.L., Goessling, W., Iterative use of nuclear receptor Nr5a2 regulates multiple stages of liver and pancreas development, 108-23, Copyright (2016) with permission from Elsevier. Full text @ Dev. Biol.