- Title
-
Neuromuscular regulation in zebrafish by a large AAA+ ATPase/ubiquitin ligase, mysterin/RNF213
- Authors
- Kotani, Y., Morito, D., Yamazaki, S., Ogino, K., Kawakami, K., Takashima, S., Hirata, H., Nagata, K.
- Source
- Full text @ Sci. Rep.
|
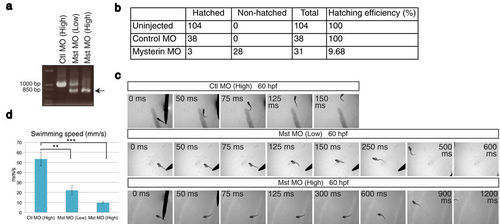
Suppression of mysterin-α leads to the failure of hatching and motor deficits in zebrafish. (a) Examination of the knockdown efficiency in morphants. Treatment with a low (1.7 ng) or high (5.1 ng) dose MO targeting mysterin-α (Mst) confirmed dose-dependent suppression of mysterin gene splicing at 2 dpf, as determined by RT-PCR. The lower band represents the products of impaired splicing (arrow). (b) Hatching defects in mysterin morphants. Morphants injected with a high dose of MO exhibit a significant hatching failure at 3 dpf. (c) Escape response to tactile stimulation at 60 hpf. Injection of a MO targeting mysterin reduces the movement of larvae in a dose-dependent manner. (d) Quantification of the swimming speed (mm/s) measured in 6 control morphants, 7 low-dose morphants, and 5 high-dose morphants at 60 hpf. **P < 0.01; ***P < 0.001. EXPRESSION / LABELING:
PHENOTYPE:
|
|
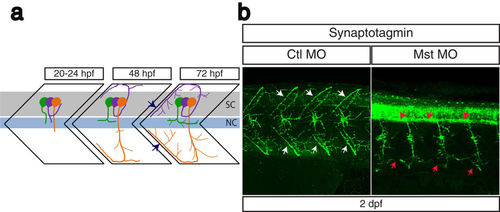
Suppression of mysterin-α leads to impaired projection of primary motoneurons during early development. (a) Schematic representation of motoneuron projection in somites during the early development of zebrafish. Primary CaP (orange), MiP (purple), and RoP (green) motoneurons project axons from the spinal code (sc). Each primary motoneuron projects its axon to a different area of muscle. In particular, intersegmental axonal projection of MiP and CaP motoneurons is indicated by blue arrows. The notochord is indicated by nc. (b) Motoneuron projection in the trunk region of a mysterin-α-suppressed animal (high dose). Primary motoneurons at 2 dpf are stained with an anti-synaptotagmin antibody (znp-1). The control animal exhibits proper projection of CaP and MiP motoneurons along the myotome segment and branched axons into intersegmental areas (white arrows). The morphant exhibits immature projection of CaP and MiP motoneurons along the myotome segment and reduced branched axons (red arrows). |
|
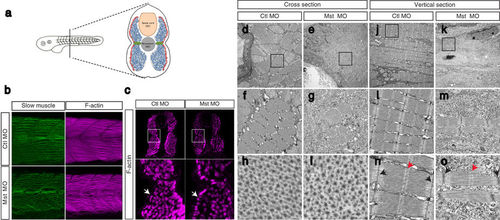
Suppression of mysterin-α leads to impaired muscle fiber formation in fast muscle. (a) Muscular composition of zebrafish embryos at 2 dpf. Slow muscle cells (red) form a monolayer structure beneath the skin. By contrast, fast muscle cells (blue) occupy the deeper region. MPCs (green) are located lateral to the notochord. (b) Fast and slow muscle structures. Fast and slow muscles in the trunk are stained with phalloidin, which recognizes actin fibers of both muscle types, and an anti-myosin heavy chain antibody (F59), which labels slow myosin fibers. F59 labeling revealed normal slow muscle fibers in control and mysterin morphants (left panels). Phalloidin labeling revealed an impaired fast muscle structure in morphants (right panels). (c) Cross-section of the trunk region. Slow and fast muscle structures are stained with phalloidin at 2 dpf. The density of F-actin fibers in the fast muscle region is severely decreased in morphants treated with a low dose of MO. White arrows indicate the slow muscle monolayer, which appears to be normal in morphants treated with a low dose of MO. (d–o) EM of the muscle structures of control animals and morphants treated with a low dose of MO at 2 dpf. Cross-sections (d–i) and vertical sections (j–o) of a control animal and morphant. The orientation of actin and myosin is shown in (h–i). The sarcomeric structure is shown in (n–o). The M-line and Z-disk are indicated by red and black arrows, respectively. |
|
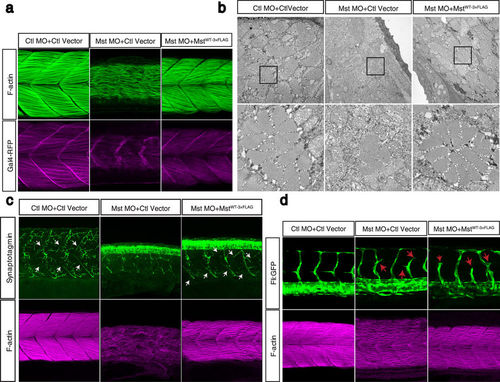
Fast muscle-specific expression of human mysterin restores the fast muscle malformation in morphants. (a) Ectopic expression of human mysterin restores the morphology of fast muscle in mysterin morphant larvae. Labeling of F-actin shows the formation of robust muscle fibers upon ectopic expression of mysterin in fast muscle, but not in the control. Expression of RFP driven by the UAS promoter shows that these larvae carry the GAL4FF-expressing transgene in fast muscle. (b) EM observation of restored fast muscle in the trunk. The lower panels show magnified images. (c) Projection of primary motoneurons in somites. Projection of motoneurons is impaired in mysterin-α morphants and restored by fast muscle-specific expression of human mysterin (white arrows). (d) Effect of suppression of mysterin-α and fast muscle-specific expression of human mysterin on vascular formation. Human mysterin tagged with a 3×FLAG epitope (WT-3×FLAG) is expressed in transgenic zebrafish that express GFP in vascular endothelial cells. Green (GFP) and magenta (F-actin) show trunk vessels and fast muscle, respectively. EXPRESSION / LABELING:
PHENOTYPE:
|
|
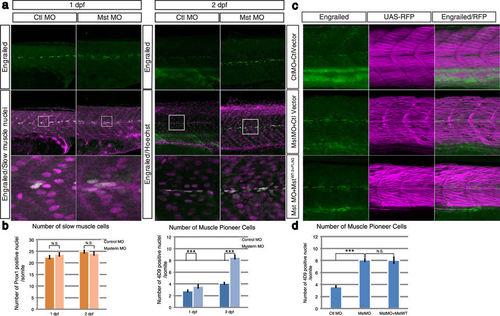
Suppression of mysterin-α significantly increases the number of MPCs. (a) Increased number of MPCs upon suppression of mysterin-α. MPCs in the trunk region are stained with an anti-engrailed antibody (green). The nuclei of slow muscle cells at 1 dpf and all cells at 2 dpf are stained with an anti-prox1 antibody (magenta, left panels) and Hoechst (magenta, right panels), respectively. The morphant has a significantly increased number of MPCs at 2 dpf but not at 1 dpf. (b) Quantification of slow muscle cells and MPCs. The left panel shows the mean number of nuclei of slow muscle cells (prox1-positive) within 20, 34, 49, and 33 somites of control animals at 1 dpf, mysterin-α morphants at 1 dpf, control animals at 2 dpf, and mysterin-α morphants at 2 dpf, respectively. The right panel shows the mean number of slow muscle nuclei (engrailed-positive) within 43, 52, 20, and 34 somites of control animals at 1 dpf, mysterin-α morphants at 1 dpf, control animals at 2 dpf, and mysterin-α morphants at 2 dpf, respectively. Error bars represent the standard deviation. N.S.: not significant. ***P < 0.001. (c) Effect of fast muscle-specific expression of human mysterin on the number of MPCs. Human mysterin tagged with a 3×FLAG epitope (WT-3×FLAG) is expressed in embryonic fast muscle using the Tol2 and GAL4/UAS systems. RFP expression is driven by fast muscle-specific GAL4FF and is thus an indicator of GAL4 activity and fast muscle morphology (See Methods). The number of engrailed-positive nuclei (green) in morphants is unchanged by fast muscle-specific expression of human mysterin. (d) Quantification of MPCs shown in (c). The panel shows the mean number of nuclei of MPCs (engrailed-positive) within 64, 29, and 25 somites of control animals, mysterin-α morphants, and human mysterin-expressing morphants, respectively, at 2 dpf. Error bars represent the standard deviation. N.S.: not significant. ***P < 0.001. |
|
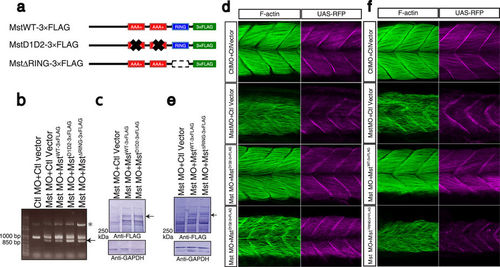
Indispensable roles of the AAA+ ATPase and ubiquitin ligase activities of mysterin. (a) Schematic representation of mysterin enzymatic mutants. D1D2 contains four mutations at conserved amino acids in the two AAA+ ATPase modules, resulting in inactivation of ATPase activity and blockade of oligomer formation. ΔRING lacks the entire RING finger domain, which eliminates ubiquitin ligase activity. (b) Examination of the knockdown efficiency in morphants by RT-PCR. The lower bands represent the impaired splicing caused by the MO (arrow). (c) Ectopic expression of human wild-type and D1D2 mutant mysterin examined by Western blotting using an anti-FLAG antibody. Intact mysterin is indicated by arrow (Also see Supplementary Fig S4b). GAPDH is the loading control. (d) Restoration of fast muscle morphology in mysterin morphants by ectopic expression of wild-type human mysterin, but not of the ATPase mutant (D1D2-3×FLAG). Fast actin fibers are labeled with phalloidin (green). RFP expression is driven by fast muscle-specific GAL4FF and is thus an indicator of GAL4 activity. (e) Ectopic expression of human wild-type and ΔRING mutant mysterin examined by Western blotting using an anti-FLAG antibody. Mysterin band (591 kDa) is indicated by an arrow (Also see Supplementary Fig S4b). GAPDH is the loading control. (f) Restoration of the fast muscle anomaly by expression of wild-type human mysterin, but not of the ubiquitin ligase mutant (ΔRING-3×FLAG). Fast actin fibers are labeled with phalloidin (green). RFP expression is driven by fast muscle-specific GAL4FF and is thus an indicator of GAL4 activity. |
|
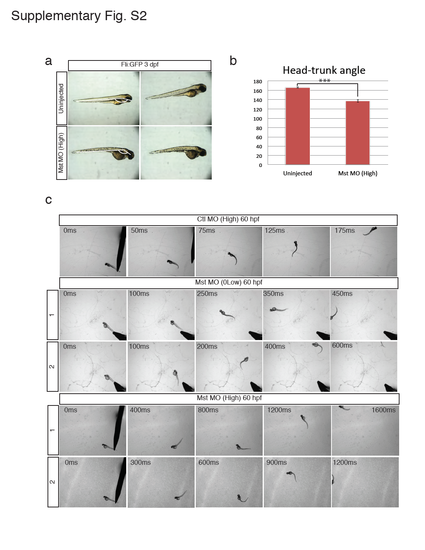
Morphology and motor function of the mysterin morphant. (a and b) Observation of mysterin morphants at 3 dpf by stereoscopic microscopy. Development of morphants treated with a high dose of the MO targeting mysterin-α is slightly delayed in comparison with that of control embryos. Control embryos and morphants showed head-trunk angles of 166.4° ± 1.2° (n=14) and 137.1° ± 2.6° (n=10), respectively, suggesting significant delay (approximately 20 hr) in embryogenesis. ***P < 0.001. Animals carry the fli:EGFP transgene, although vascular endothelial cells were not assessed in this figure. (c) Additional successive images of embryonic swimming at 60 hpf. Tracked embryos are independent of those shown in Fig. 1c. |
|
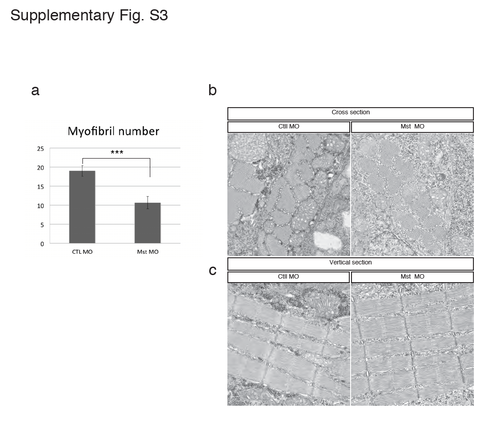
Electron micrograph of the slow muscle structure in the mysterin morphant. (a) Quantification of myofibril numbers in 5 μm2 square of cross-sectionnal electron micrograph. Control and mysterin MOs show 19.0 ± 1.4 (n=12) and 10.6 ± 1.6 (n=11) myofibrils in 5 μm2 square, respectively. ***P < 0.001. Cross-section (b) and vertical section (c) of transgenic animals (gSA2AzGFF598A) that express Gal4FF in fast muscle. Slow muscle is identified by its distribution and the thickness of the Z-disk. Treatment with a low dose of the MO targeting mysterin-α does not affect slow muscle structures. |
|
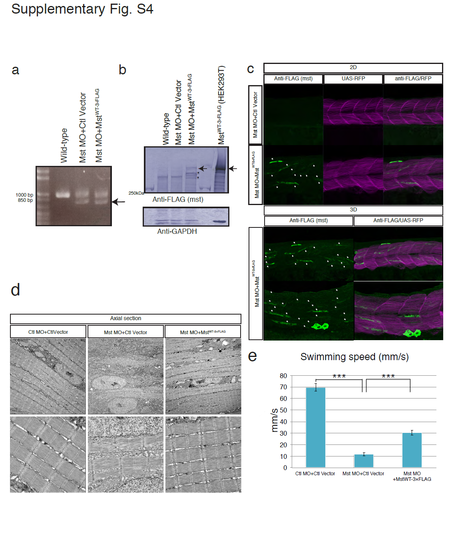
Fast muscle-specific expression of human mysterin restores the fast muscle malformation and motor deficits of mysterin morphants. (a) RT-PCR analysis of the knockdown efficiency (2.1 ng of MO) in morphants. The lower bands represent the impairment of splicing. (b) The expression level of human mysterin in fast muscle was examined by Western blotting using an anti-FLAG antibody. Protein extracts of Mst-3×FLAG-expressing HEK293 cells1 was loaded for an indication of mysterin products (591kDa; arrow). Mysterin-derived fragments which are not observed in control lanes are indicated by stars. GAPDH is the loading control. (c) Immunostaining of Mst-3×FLAG that is specifically expressed in fast muscle. Control and human mysterin-expressing morphants are stained using an anti-FLAG antibody (white arrows). A single layer and superimposed 3D image are shown separately. RFP is a marker of GAL4 activity. Human mysterin-3×FLAG is distributed in the cytosol, which is consistent with our previous observation in human embryonic kidney 293 cells1. (d) EM of vertical sections of human mysterin-expressing morphants. Fast muscle-specific expression of human mysterin-3×FLAG rescues malformation of muscle fibers. Lower panels are magnified images. (e) Swimming motility (mm/s) of human mysterin-expressing morphants. |
|
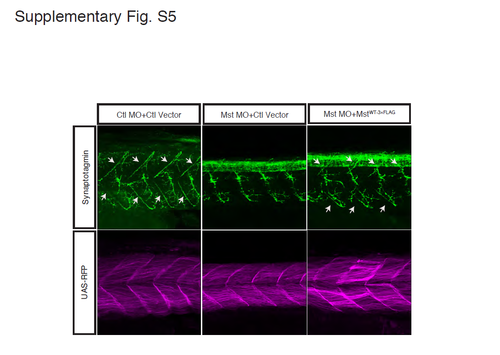
Projection of motoneurons is restored by fast muscle-specific expression of human mysterin. Projection of motoneurons is impaired by the MO targeting mysterin-α and is partially restored by fast muscle-specific expression of human mysterin. Motoneurons are labeled using an anti-synaptotagmin antibody. RFP shows Gal4 activity. White arrows show baseline and restored intersegmental projections. The stronger staining of synaptotagmin at the notochord is due to a developmental delay caused by suppression of mysterin-α and is not restored by fast muscle-specific expression of human mysterin. |
|
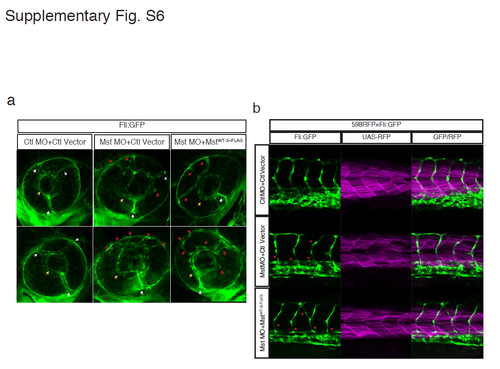
A morphological defect of blood vessels in mysterin morphants is not restored by fast muscle-specific expression of human mysterin. (a) Visualization of the vascular structure in the fliEGFP transgenic line. Although three branches of the nasal ciliary artery (white arrows) drain into the inner optic circle (IOC; yellow arrows) in control animals, multiple aberrant vessels drain into the IOC (red arrows) in morphants. This phenotype is not restored by fast muscle-specific expression of human mysterin. (b) Guidance of trunk vessels is severely impaired by mysyerin-α knockdown (red arrows). This anomaly is not restored by fast muscle-specific expression of human mysterin. RFP shows fast muscle-specific Gal4 activity. |
|
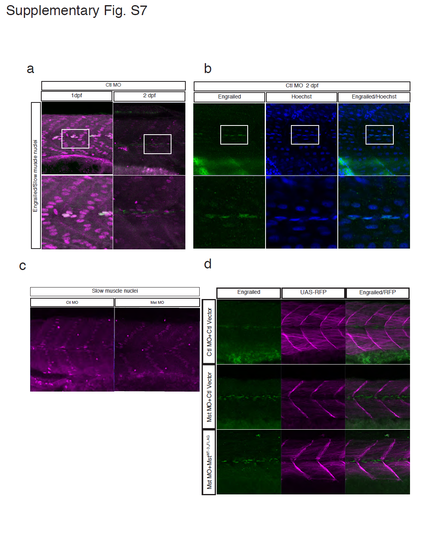
Characterization of a previously unidentified subpopulation of MPCs and the failure to recover MPCs by fast muscle-specific expression of human mysterin. (a) Co-staining of slow muscle nuclei (prox1: magenta) and MPC nuclei (engrailed: green) in control embryos at 1 and 2 dpf. Although MPCs are known as a subpopulation of slow muscle cells2, prox1-negative and engrailed-positive cells appear at 2 dpf. (b) Co-staining of MPC nuclei (engrailed: green) and all nuclei (Hoechst: blue) in control embryos at 2 dpf. The proper nuclear distribution of engrailed is confirmed by co-staining of total nuclei (Hoechst: blue). The number of engrailed-positive cells (MPCs) at 2 dpf was counted. (c) The MO targeting mysterin-α does not alter the number of prox1-positive slow muscle cells at 2 dpf. Quantification is shown in Fig. 5b. (d) MPCs are stained with an anti-engrailed antibody (green). The morphant has a significantly increased number of MPCs at 2 dpf, which is not affected by fast muscle-specific expression of human mysterin. RFP shows fast muscle-specific GAL4 activity. |