- Title
-
Zebrafish transgenic line huORFZ is an effective living bioindicator for detecting environmental toxicants
- Authors
- Lee, H.C., Lu, P.N., Huang, H.L., Chu, C., Li, H.P., Tsai, H.J.
- Source
- Full text @ PLoS One
|
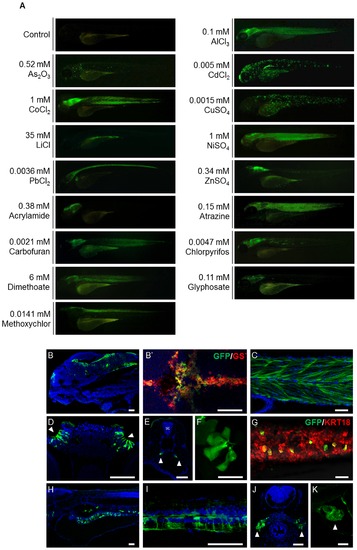
The GFP signals shown in huORFZ embryos are distinctly responsive to various stresses. (A). Various heavy metal-containing chemicals, including AlCl3, As2O3, CdCl2, CoCl2, CuSO4, LiCl, NiSO4, PbCl2 and ZnSO4, or different EDCs, including acrylamide, atrazine, carbofuran, chlorpyrifos, dimethoate, glyphosate and methoxychlor, were used individually to treat huORFZ embryos at 72 hpf. The concentrations of each heavy metal ion or EDC used were indicated on each panel. GFP expression patterns were observed at 96 hpf. The percentages of the representative patterns among the treatment groups are labeled on the images. (B-K). Selected GFP expression patterns were imaged in detail under confocal microscopy. For AlCl3-treated huORFZ embryos, GFP signals were observed in the brain (B) and muscle (C). Through immunostaining of antibody against GS, it was observed that only the glial cell lineages in the brain of embryos expressed GFP (B2 red labeling). For CdCl2-treated huORFZ embryos, GFP signals were detected in the olfactory epithelium (D; arrowhead), pronephric ducts (E; arrowhead), skin and lateral line system (F). For CuSO4-treatment, embryos obtained from crossing huORFZ to Tg(-2.9krt18:RFP) were used. When treated with CuSO4, GFP-expressing skin cells were always also krt18:RFP-expressing, indicating a keratinocyte lineage (G). For PbCl2-treated huORFZ embryos, the GFP-positive tissues included the spinal cord (I) and kidney (J; arrowhead). Interestingly, when either LiCl or Atrazine was used to treat the huORFZ embryos, GFP signals were observed in the intestine (H) and heart (K; arrowhead), respectively. (A, B, C, F – I, K) are lateral views with anterior to the left. (B2) is dorsal view with anterior to the left. (D, E, J) are transverse view with posterior to the top. SC: spinal cord. BR: brain. The scale bar in F is 20 µm; all other scale bars are 50 μm. |
|
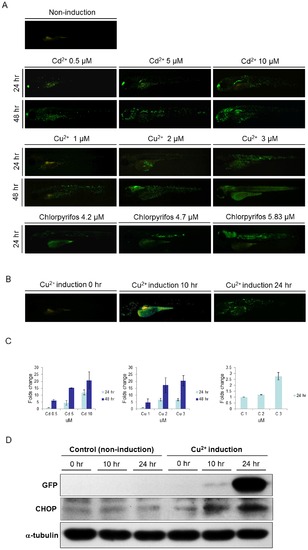
The intensity of GFP signal was positively correlated with the strength of stress and the expression of endogenous Ddit3. (A). At 72 hpf, huORFZ embryos were exposed to different concentrations of Cadmium (Cd2+), Copper (Cu2+), and chlorpyrifos, as indicated, and fluorescence signals were observed at 96 and 120 hpf. Mock group was treated with water containing DMSO which was added to the concentration representing the DMSO in the chlorpyrifos treatment group. As the chemical concentrations and incubation times increased, the GFP signals also increased. All images are lateral views with anterior to the left. (B). GFP expression patterns of huORFZ embryos after treatment with 0.1 mg/L (1.5 μM) of Cu2+ from 72 hpf for 10 hr and 24 hr, as indicated. All images are representative with the percentage among treatment groups labeled. All images are lateral views with anterior to the left. (C). The semi-quantification analysis based on fluorescent images. Both increased toxicant concentration and prolonged treatment time resulted in increased GFP signal intensity in huORFZ embryos. Note that the readings of each chemical treatment were normalized to the lowest dosage group of the same chemical. Thus, the signal values from different chemical treatments were not comparable. (D). The expression of endogenous Ddit3 in huORFZ embryos positively correlates to the signal strength of stress-induced GFP. Total cell lysates were prepared and analyzed by Western blot with specific antibodies to exogenous GFP and to the ER stress protein Ddit3. α-tubulin served as a loading control. |
|
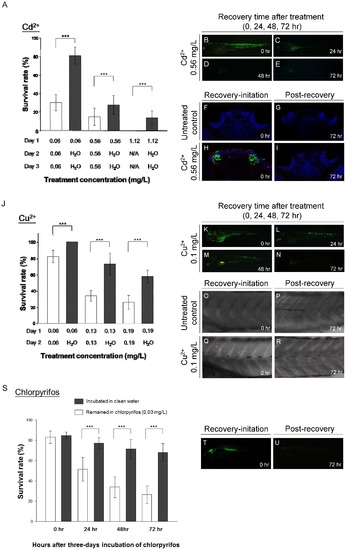
GFP expression in huORFZ as signals of cell under acute and chronic toxic stresses. At 72huORFZ embryos were treated with Cadmium (Cd2+; A-I) or Copper (Cu2+; J-R). (A). The survival rate of huORFZ embryos incubated in Cd2+ with different concentrations and treatment time. Open bars indicate huORFZ embryos that remained in the Cd2+ solution throughout the experiment; black bars indicate the embryos removed for recovery in clean water after 24 hr of Cd2+ treatment. N/A indicates that prolonged treatment was not conducted as a result of 100% lethality. (B-E). GFP signals immediately or 24, 48 and 72 hr after 24 hr of Cd2+ treatment. (F-I). TUNEL assay at the olfactory epithelium immediately or 72 hr after 24 hr of Cd2+ treatment. Red color represents TUNEL assay; Green color represents GFP signal; and Blue color represents DAPI staining. (J). The survival rates of huORFZ embryos incubated in Cu2+ with different concentrations and treatment times. The experimental strategy was the same as A-I. (K-N). GFP signals immediately or 24, 48 and 72 hr after 24 hr of Cu2+ treatment. (O-R). TUNEL assay at the skin immediately or 72 hr after 24 hr of Cu2+ treatment. (S-U). For the chronic toxicity test, 72 hpf huORFZ embryos were treated with chlorpyrifos with a concentration (86 nM) below WHO guidelines. (S). The survival rates of the embryos treated with chlorpyrifos for zero to three days. (T, U). GFP signals immediately or 72 hr after three days of chlorpyrifos treatment. (F-I). are transverse section with dorsal to the top. All other images are lateral views with anterior to the left. For each treatment (each bar in A, J and S), n = 100 embryos evenly distributed in five repetitions. |
|
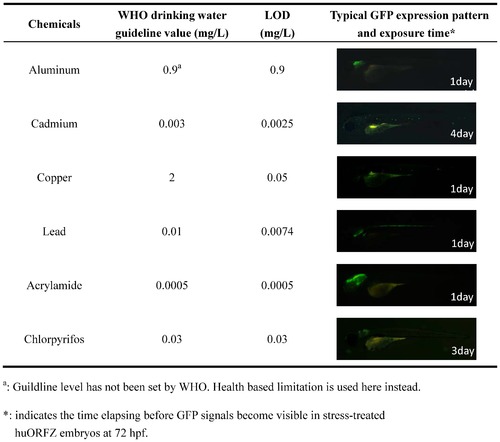
The limit of detection (LOD) of huORFZ embryos can reach WHO guideline values for various heavy metals and endocrine-disrupting chemicals (EDCs). LOD was defined as the lowest tested concentration that led to detectable GFP signals in more than 80% of the treated embryos after one to four days of treatment. For each kind of treatment, a representative image of a huORFZ embryo treated with the chemical at the LOD concentration for the period of time indicated was presented. All images are lateral views with anterior to the left. |
|
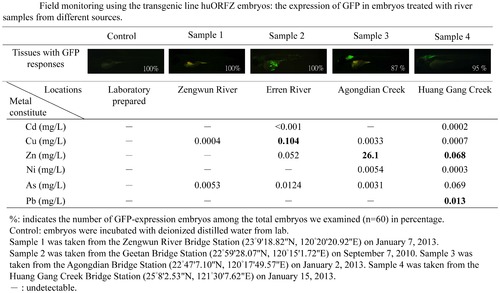
Embryos derived from transgenic line huORFZ provide true signals of a contaminated aquatic environment. The GFP fluorescent signal intensities induced in huORFZ embryos showed responses relative to different river samples collected from local waterways. In Sample 1, no GFP signal was observed in huORFZ embryos, consistent with WHO water safety standards. In Sample 2, the GFP response in skin tissue of huORFZ embryos indicated potential copper pollution. In Sample 3, GFP signals in the brain of huORFZ embryos corresponded to embryonic toxicity consistent with high Zn2+ levels. Finally, in Sample 4, the GFP response shown in CNS of huORFZ embryos was attributed to the presence of multiple pollutants, such as Zn2+ and Pb2+. All images are lateral views with anterior to the left. |
|
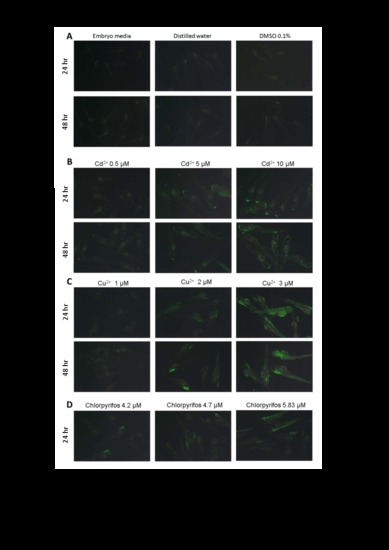
Images of larger field of view are used to demonstrate the general patterns and individual variability of huORFZ embryos treated with different heavy. (A) huORFZ embryos were treated with embryo media, ddH2O, or DMSO for 24 and 48 hr starting at 72 hpf. (B-D) The effects of different treatment times (24 and 48 hr) with different concentrations of cadmium (0.5, 1, and 5 μM), copper (1, 2 and 3 μM) and chlorpyrifos (4.2, 4.7, and 5.83 μM) on huORFZ embryos. For chlorpyrifos treated group, at 48 hr treatments are 100% lethal. All images were taken under the Leica MZ FLIII microscope with 2x objective. All images were taken under the same exposure time, iso value and other camera settings. |
|
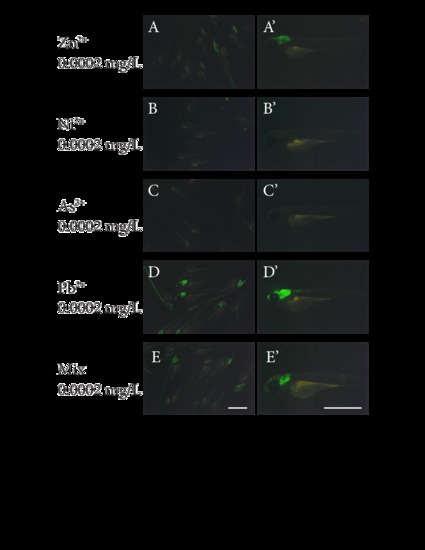
The four major metal pollutants found in the river water sample 4 are sufficient to induce huORFZ embryos to express the GFP signal similar to what was caused by river water sample 4. huORFZ embryos were treated with water containing (A, A2) Zinc, (B, B2) Nickel, (C, C2) Arsenic, or (D, D2) Lead ion individually, or (E, E2) the water containing all four pollutants. The left panel (A, B, C, D, E) demonstrates group images taken under 2x objective while the right panel (A2, B2, C2, D2, E2) contains the images of one representative embryo of each group, taken under 4x objective. Right panel images are lateral views with anterior to the left. All scale bars are 1 mm. |
|
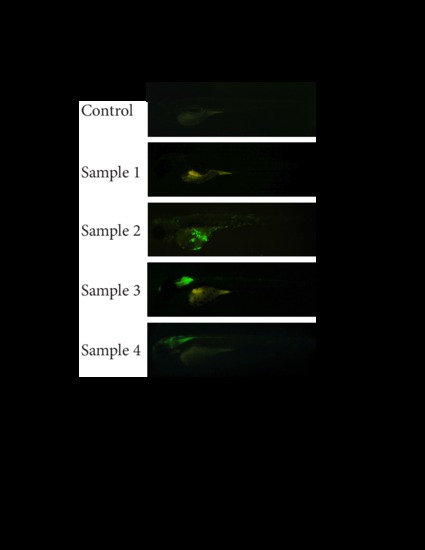
Higher resolution images of the images presented in Figure 5. All images are exactly the same as in Figure 5, only in larger format. |