- Title
-
Fgf3 and Fgf10a work in concert to promote maturation of the epibranchial placodes in zebrafish
- Authors
- McCarroll, M.N., and Nechiporuk, A.V.
- Source
- Full text @ PLoS One
|
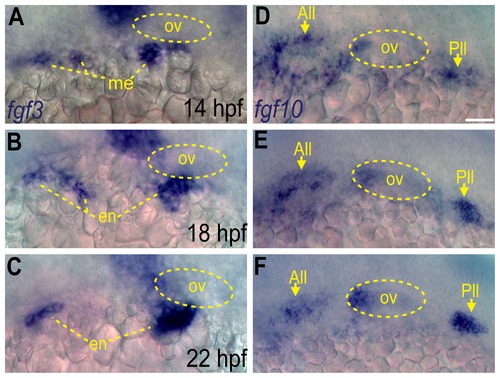
Fgf3 and Fgf10a are expressed during epibranchial placode formation. (A-C) In situ hybridization reveals presence of fgf3 transcript in the mesoderm at 14 hpf (A), and then in the endoderm at 18 (B) and 22 hpf (C). (D-F) In situ hybridization reveals presence of fgf10a transcript in the anterior and posterior lateral line (arrows) and the anterior portion of the otic vesicle at 14 (D),18 (E) and 22 hpf (F). Otic vesicle is outlined by a dotted line in (A-F). Abbreviations: ov, otic vesicle; me, mesoderm; en, endoderm; All, anterior lateral line; Pll, posterior lateral line. Scale bar: 50 μm. EXPRESSION / LABELING:
|
|
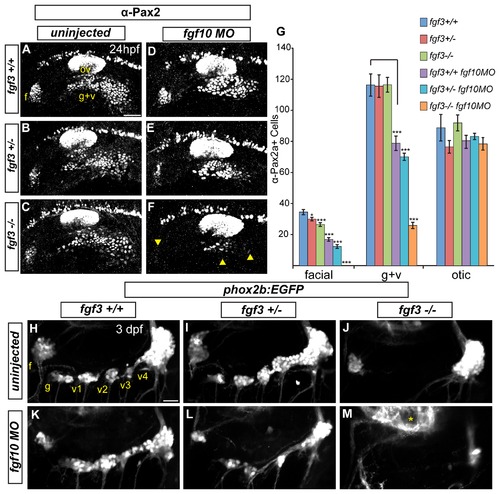
Fgf3 and Fgf10a are required for maturation of epibranchial placodes and development of the epibranchial ganglia. (A-C) Confocal projections showing Pax2a expression in wild-type (A) fgf3+/- (B) and fgf3-/- (C) embryos at 24 hpf. (D-F) Confocal projections showing Pax2a expression in 24-hour old wild-type (D) fgf3+/- (E) and fgf3-/- (F) embryos injected with fgf10a-MO. Note the significant loss of Pax2a expression in the EB placodes (F; arrowheads). (G) Pax2a+ cell number in the facial, glossopharyngeal/vagal, and otic placodes for conditions in (A-F). Note the complete loss of Pax2a+ cells in the facial placode and a 4.5 fold reduction in the glossopharyngeal/vagal placode in fgf3-/-;fgf10a-MO embryos (ANOVA multiple comparison with Bonferroni’s correction; *P<0.05; ***P<<0.001; Error bars: standard error of mean; n=11 embryos per condition). (H-M) Confocal projections of 3 dpf wild-type (H), fgf3+/- (I) and fgf3-/- (J) embryos and wild-type (K), fgf3+/- (L) and fgf3-/- (M) embryos injected with fgf10a-MO. All embryos contain TgBAC(phox2b:EGFP) which marks EB ganglia. Note the loss of EGFP expression in the glossopharyngeal and three small vagal ganglia in fgf3-/- embryos (J) and the complete loss of EGFP expression in all EB ganglia in fgf3-/-;fgf10a-MO with the exception of a few EGFP+ cells in the region of the large vagal ganglion (M). Asterisk marks hindbrain neurons also expressing TgBAC(phox2b:EGFP). Abbreviations: f, facial placode (A) or facial ganglia (H); ov, otic vesicle; g+v, glossopharyngeal/vagal placode; g, glossopharyngeal ganglia; v1-v3, small vagal ganglia 1-3; v4, large vagal ganglion. Scale bars: 50 μm (A); 25 μm (H). EXPRESSION / LABELING:
PHENOTYPE:
|
|
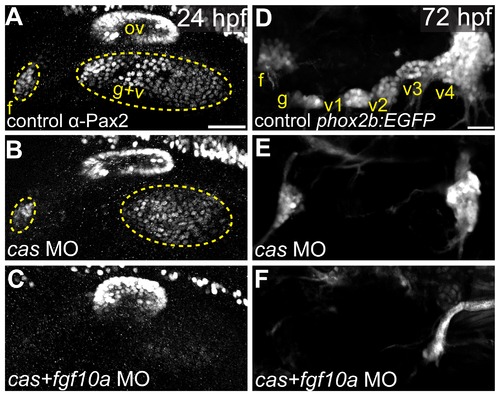
Fgf10a and endodermally derived Fgf3 cooperate during EB placode formation. (A-C) Confocal projections of Pax2a expression in control (A), casanova-MO injected (B), or casanova/fgf10a-MO coinjected (C) embryos at 24 hpf. (D-F) Confocal projections of 72 hpf TgBAC(phox2b:EGFP) in control (D), casanova-MO (E), or casanova/fgf10-MO (F) embryos. Note a complete loss of Pax2a and EGFP expression in EB placodes and ganglia, respectively, in casanova/fgf10a-MO embryos (C and F). Abbreviations: f, facial placode (A) or facial ganglia (D); ov, otic vesicle; g+v, glossopharyngeal/vagal placode; cas, casanova; g, glossopharyngeal ganglia; v1-v3, small vagal ganglia 1-3; v4, large vagal ganglion. Scale bars: 50 µm (A); 25 μm (D). EXPRESSION / LABELING:
PHENOTYPE:
|
|
The anterior lateral line is the tissue source of Fgf10a responsible for facial placode development. (A-D) confocal projections of Tg(pax2a:EGFP) zebrafish embryos (green) analyzed for Pax2a expression (magenta) at 14 (A), 18 (B), 21 (C), and 24 hpf (D). The presumptive facial placode is outlined in yellow as it condenses between 18 and 24 hpf. Insets show co-expression of Pax2a and Tg(pax2a:EGFP) at 14 hpf (A); by 18 hpf, however, Pax2a expression is absent in anterior lateral line precursors, while Tg(pax2a:EGFP) maintains expression in these cells (B). (E-E′′) Live confocal projection of a 12 hpf Tg(pax2a:Kaede) zebrafish embryo (E) with the anterior portion of the pax2a:Kaede+ domain photoconverted from green to red emission (E′) overlay (E′′). (F-F′′) Composite image of the same photoconverted embryo from (E) at 24 hpf analyzed for Pax2a expression (F) and cyan in (F′′) and photoconverted Kaede (F′) and red in (F′′). Note absence of Kaede positive cells in the facial placode. (G, H) In situ hybridization of eya1 in 52 hpf zebrafish embryos reveals proper neuromast deposition in control (G) and a failure of deposition and elongation of the anterior lateral line in fgf3-/-;fgf10-MO embryo (H; arrowheads). (I, J) Lateral views of the 24 hpf fgf3+10 morphant embryo showing the side that received wild-type donor cells (green) as well as the contralateral control side that did not receive donor cells. Pax2a expression is visualized by immunolabeling (magenta). Note partial rescue of the facial placode when wild-type donor cells were present in the presumptive anterior lateral line (J; arrowhead). (K, L) Quantification of Pax2a+ cells reveals a significant increase in the number of Pax2a+ cells in the facial (K) and glossopharyngeal and vagal placodes (L) of the transplanted sides versus contralateral sides (Wilcoxon matched-pairs signed rank test: **P<0.01; error bars: standard error of mean; n=8 embryos). Abbreviations: f, facial placode; g+v glossopharyngeal/vagal placode; ov, otic vesicle. Scale bars: 50 μm (A, I); 25 μm (G). EXPRESSION / LABELING:
PHENOTYPE:
|
|
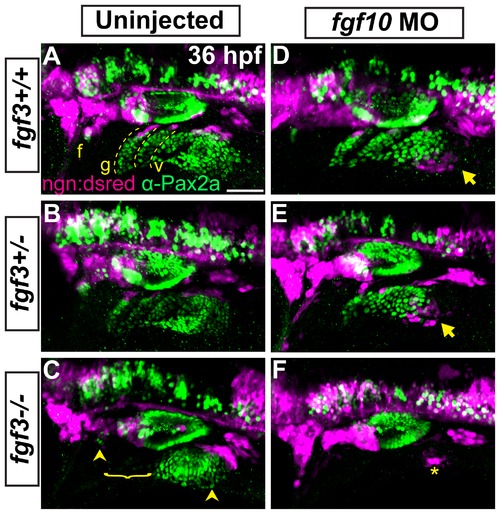
Fgf3 and Fgf10a are required for placode maturation and neurogenesis. (A-F) Uninjected and fgf10a-MO injected progeny from fgf3+/-;TgBAC(neurog1-DSRed) crosses were collected at 36 hpf and immunolabeled for Pax2a and DSRed to visualize EB placodes and migrating neuroblasts, respectively. Wild-type (A) and fgf3+/- (B) panels shows neurog1:DSRed+ cells undergoing neurogenesis (magenta) at the dorsal aspect of the mature Pax2a+ EB placodes (green; dotted line). fgf3-/- embryos show a loss of properly formed Pax2a+ EB placodes in the region of the prospective glossopharyngeal and three small vagal ganglia and a concurrent loss of neurog1:DSRed+ cells in this region (C; bracket); however, the facial and large vagal placode/ganglia are still present (arrowheads). Analysis of fgf10a-MO injected wild-type (D) and fgf3+/- (E) embryos reveal ectopic neurogenesis as marked by neurog1:DSRed+ cells ventral to the posterior most aspect of the vagal placode (arrow). fgf3-/-;fgf10-MO embryos show a complete loss Pax2a expression in EB placodes and an absence of neurogenesis, except for a few neurog1:DSRed+ cells near the region of the large vagal ganglia (F; asterisk). Abbreviation: ngn:dsred, TgBAC(neurog1-DSRed); f, facial placode; g, glossopharyngeal placode; v, vagal placodes. Scale bar: 50 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
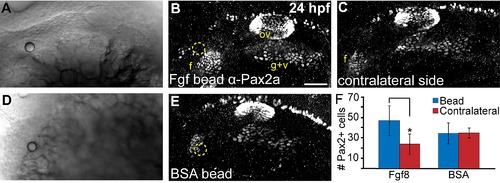
Local Fgf activity is sufficient to expand the facial placode. (A-E) Twenty-four hour old zebrafish embryos that received heparin beads soaked in either recombinant Fgf8 (A-C) or BSA (D,E) were immunostained for Pax2a expression and imaged using either transmitted light (shows site of bead implantation in A, D) or confocal microscopy in (B,E; bead is outlined in yellow). Note the expansion of the facial placode (f) near the Fgf8-soaked bead (B) compared to contralateral control of the same embryo (C). (F) Quantification of Pax2a+ cells in the facial placode revealed a 2 fold increase in the facial placode in embryos that received an Fgf8 soaked bead compared to the contralateral side (Wilcoxon matched-pairs signed rank test; *P<0.05; error bars: standard error of mean; n=5 embryos/condition). |
|
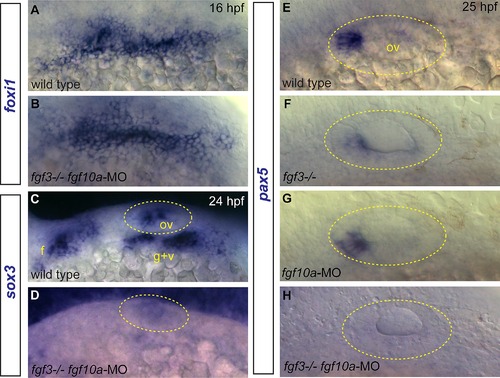
Effects of Fgf3+10a loss on development of EB and otic placodes. (A, B) foxi1 expression detected by in situ hybridization in 16 hpf zebrafish embryos reveals no difference in distribution of EB placode precursors in control (A) and fgf3-/-;fgf10a-MO (B) conditions. (C, D) sox3 expression detected by in situ hybridization in 24 hpf zebrafish embryos. Control shows expression of sox3 transcripts in the otic vesicle (outlined in yellow), and the EB placodes (C); sox3 expression is lost in these structures in the fgf3-/-;fgf10a-MO embryo (D). (E-H) pax5 expression detected by in situ hybridization in 25 hpf embryos. Control conditions show expression of pax5 in the anterior portion of the otic vesicle (E). Whereas only partial loss of pax5 was observed in fgf3-/- (F) or fgf10a-MO (G) embryos, complete loss of pax5 expression was observed in fgf3-/-;fgf10a-MO embryo (H). Abbreviations: f, facial placode; g+v glossopharyngeal/vagal placode; ov, otic vesicle. |
|
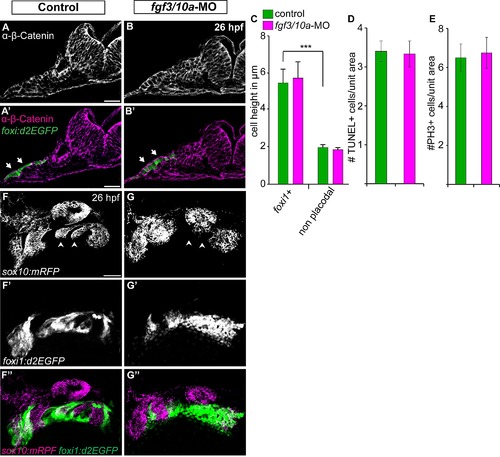
Fgf3 and Fgf10a are not required during EB placode induction, proliferation, and survival, but they are required for the EB placode and NC interaction. (A, B) Confocal projections of TgBAC(foxi1:d2EGFP) (green) 26 hpf zebrafish embryos immunostained for β-Catenin (magenta). Images show unilateral transverse sections at the level of the glossopharyngeal/vagal placode (arrows). Note columnar morphology of the epithelial cells lateral to the otic vesicle in control (A) and fgf3+10a-MO embryos (B). (C) Average cell height of foxi1:d2EGFP+ cells measured in μm was unchanged in fgf3/10a-MO injected embryos compared to controls, measurement non-placodal cells medial to the foxi1+ cells are significantly shorter (Error bars: standard error of mean. ANOVA multiple comparison with Sidak′s correction; ***P<<0.001; ne25 cells from 5 individual embryos per condition). (D, E) Comparison of TUNEL+ cells or PH3+ cells per unit area of the prospective EB placodes between control and fgf3+10a-MO injected 18 hpf embryos reveals no change in cell death or proliferation at this stage (ne8 embryos per condition). (F, G) Confocal projections of 26hpf embryos derived from crossing Tg(sox10(7.2):mrfp) to TgBAC(foxi1:d2EGFP) parents. Control conditions show properly formed branchial arches (F; arrowheads), and mature placodes assembling within corridor like structures (F′, F′′). In fgf3+10a-MO embryo, a subset of branchial arches is absent (G; arrowheads); however the anterior and posterior most NC derived structures are still present. Foxi1-positive placodal ectoderm is present, albeit not properly organized at this stage (G′, G′′). Scale bars: 25μm (A, A′); 50μm (F). |