- Title
-
Zebrafish eda and edar mutants reveal conserved and ancestral roles of ectodysplasin signaling in vertebrates
- Authors
- Harris, M.P., Rohner, N., Schwarz, H., Perathoner, S., Konstantinidis, P., and Nüsslein-Volhard, C.
- Source
- Full text @ PLoS Genet.
|
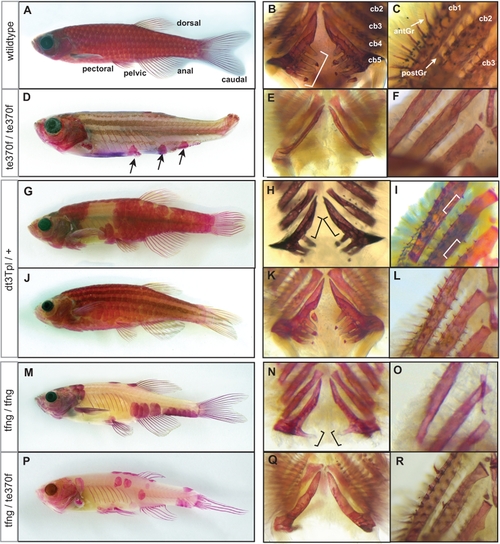
The formation of the adult dermal skeleton and pharyngeal teeth is affected in fls mutant zebrafish. A) Alizarin red-stained wild type adult zebrafish shows staining of the scales, fin rays, dermal bones of the skull as well as the pharyngeal teeth along ceratobranchial 5 (bracket, B) and (C) gill rakers along both the anterior (antGr) and posterior edge (postGr) of non-teeth bearing ceratobranchials. D) flste370f shows loss of dermal skeletal structures of the fin rays, scales and alteration in the shape of the skull. Additionally, flste370f shows loss of pharyngeal teeth (E) and gill rakers (F). G–I) The Topless allele (flsdt3Tpl) shows a dominant effect on scalation and tooth/gill raker formation while not affecting lepidotrichial growth. J–L) Expressivity of flsdt3Tpl is sensitive to a modifier in the Tü strain leading to a “weak” flsdt3Tpl phenotype; flsdt3Tpl homozygotes were phenotypically identical to flste370f (not shown). The fang allele of fls, flstfang, isolated in a non-complementation screen with flste370f, shows no effect on fin development while exhibiting partial loss of scales (M), teeth (N), and gill rakers (O). Transallelic tfang/te370f zebrafish exhibit an intermediate phenotype between homozygous fls(te370f) and fls(tfang) (P–R). EXPRESSION / LABELING:
PHENOTYPE:
|
|
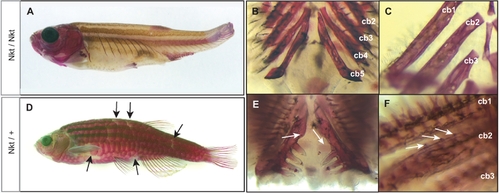
The dominant gene Nkt is phenotypically similar, however complements fls mutants. Nkt homozygotes show complete loss of scales, teeth and gill rakers resembling the fls phenotype (A–C). Heterozygous Nkt zebrafish show an intermediate phenotype of scale loss and patterning defect (arrows) while no effect on fin development is seen (D). Heterozygous Nkt also show a dominant effect on the number of teeth (arrows, E) and gill rakers (F), showing deficiencies along the posterior branchial arches and formation of rudimentary rakers along ceratobranchial 1 and 2 (arrows, F). Cb1-5, ceratobranchial bones. PHENOTYPE:
|
|
The role of edar in expression of developmental patterning genes during early scale development. Expression of edar (A, B) and eda (C, D) in early forming scales; arrowheads indicate site of expression of initial forming scales. A) edar expression above site of scale formation in 8 mm long (approximately 30 dpf juvenile fish) and in larger juveniles (9 mm; 30 dpf). C) eda expression during early scale development on the flank (8 mm) and in forming scales of older juvenile fish (10 mm, D). Expression of developmental genes bmp2b and shh in early scale development in wildtype (E, G) and flste370f (F, H) juveniles (9 mm). EXPRESSION / LABELING:
|
|
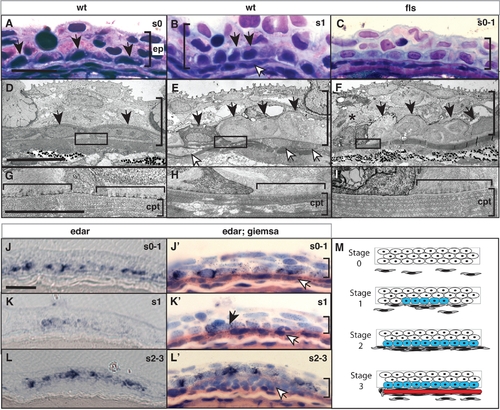
Eda signaling regulates the formation of an epidermal placode during scale development. Histological analysis of wild type (A, D, B, E) and flste370f (C, F, I) integument of 8 mm standard length. In wild type juveniles (B, E), basal epidermal cells (black arrow heads) show a heightened, and cuboidal morphology at sites of scale development as indicated by an accumulation of migrating fibroblast-like cells (white arrowheads). (H) This morphology of the epidermis is associated with a reworking of the collagen layer of the stratum compactum (cpt; [24]). This is in contrast to the flattened morphology of basal epidermal cells lateral to those of the scale placode (A, D) and underlying dense stratum compactum (G). In flste370f this basal epidermal structure is disorganized and cell morphology is disrupted (C, F) including evidence of cell death (asterisk). The lack of reworking of the collagen of the stratum compactum in the flste370f mutant is associated with retention of hemidesmosomes (horizontal bracket G–I). edar is expressed in cells of the wildtype epidermis (J, K, L). Counterstaining of the same sections confirms the expression in basal cells overlying initial accumulating fibroblasts (white arrowheads; J′, K′, L′). Expression of edar is observed prior to organization of the placode and fibroblast aggregation and maintained in cells of the epidermal placode through early scale development (J–L). M) Schematic depicting scale development and edar expression. The stages of scale development are modeled using analogous stages as described for hair development [35]; stage 0, nascent epidermis; stage 1, placode specification; stage 2, scale pocket; stage 3, matrix deposition and ossification. Blue, edar expression; red, scale formation. ep, epidermis; cpt stratum compactum. The vertical bracket demarcates the extent of the epidermis in the sections. Measurement bar equals 10 μm. EXPRESSION / LABELING:
|
|
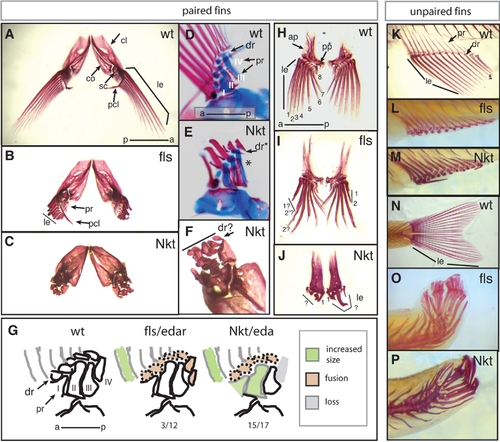
Fin development is defective in fls and Nkt mutant zebrafish. Alizarin red stained adult zebrafish fins show a drastic effect of flste370f and Nkt on development of the lepidotrichial dermal rays of both the paired and unpaired fins. A–C,F) Pectoral fins, anterior-dorsal view; D, E) double staining developing pectoral fins with alcian blue and alizarin red show early patterning of the endochondrial bones of the pectoral fin of size matched wild type and Nkt homozygotes (asterisk indicates loss of fourth proximal radial). G) effect of fls and Nkt mutants on the patterning of the pectoral fin skeleton scored as the number of specimens showing alteration in pattern or form over total analyzed. The identity of the proximal radials is noted (I–IV). H–J) Analysis of pelvic fin development in fls and Nkt mutants. Numbers denote anterior-posterior identity of the lepidotrichia. K–M) Defects in the formation of the lepidotrichia in adult anal and (N–P) caudal fin of fls (L and O) and Nkt (M and P). ap, ascending process; cl, cleithrum; co, corticoid; dr, distal radial; sc, scalpula, le, lepidotrichia; pcl, postcleithrum; pr, proximal radial. |
|
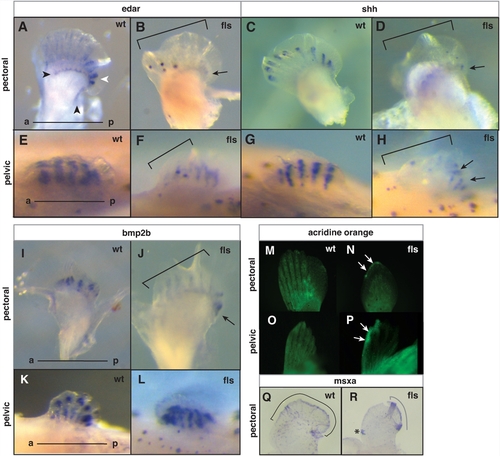
Eda signaling and the maintenance of anterior-posterior pattern in late paired fin development. Analysis of edar (A–B, E–F), shh (C–D, G–H), and bmp2b (I–L) expression in developing pectoral (A–D, –J) and pelvic fins (E–H, K–L) from 8 mm juvenile fish of wild type (A, E; C, G; I, K) and flste370f mutant fish (B, F; D, H; J, L). A–B) Arrowheads indicate two distinct patterns of edar expression in the pectoral fin: an expression that marks the posterior edge and distal region of the development of the proximal radials (black); and a posterior bias of edar expression in the forming lepidotrichia (white). Arrows point out the remaining posterior expression in mutant fins. Brackets in all panels outline anterior deficiencies in gene expression in fls mutant fins. M–P, analysis of patterns of cell death in the developing paired fins by retention of acridine orange stain. N, P) Arrows point out anterior distal regions of cell death in both pectoral and pelvic fins from the mutant; (M, N) pectoral fin and (O, P) pelvic fin respectively. Q, R) Expression of msxa in wild type and mutant pectoral fins. Region of expression outlined with brackets; asterisk marks an ectopic site of expression. EXPRESSION / LABELING:
|
|
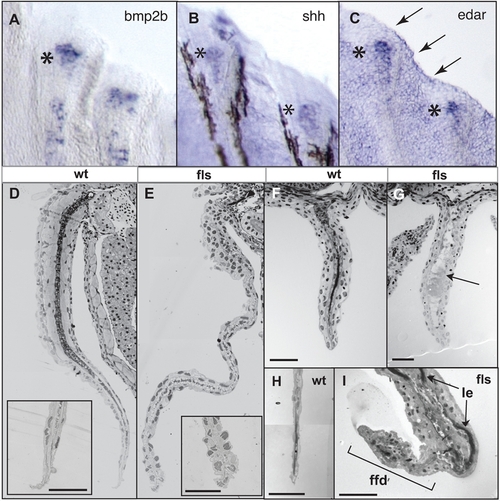
Eda signaling is required for the function of the fin fold during late fin development. Expression of bmp2b (A), shh (B) and edar (C) transcripts in developing juvenile (8 mm, 30 dpf) fin rays of the caudal fin; asterisks indicate regional expression within distal tip of developing ray; arrows in (C), expression in distal epidermis of the fin fold. D–I) Histological analysis of both paired (pectoral, D, E) and unpaired fins (anal F, G; and caudal, H, I) from flste370f and wild type siblings. flste370f fins showed a general deficiency in the maturation of the muscle and dermis of the fin (arrow G; acellular debris in anal fin of fls). Insets (D, E), tip of fin at higher magnification showing degeneration of the nuclei of the epidermis in the mutant fin. ffd, fin fold; le, lepidotrichia of the fin rays. EXPRESSION / LABELING:
PHENOTYPE:
|
|
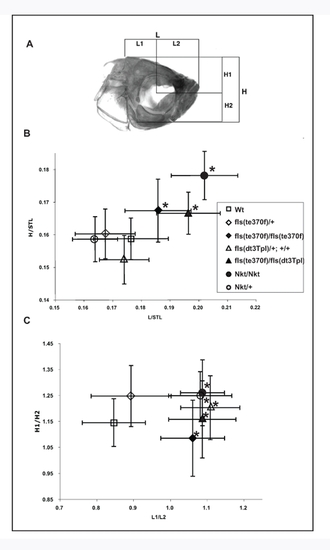
fls and Nkt alter size and proportion of the adult zebrafish skull. In addition to variations in integumentary structures, fls and Nkt exhibited a distinct change of the shape and size of the adult skull. Measurements of the absolute proportions of the adult skull, normalized for overall growth of the fish as determined by standard length, demonstrate that both fls and Nkt homozygous mutations result in overall larger skulls of the fish (flste370f/te370f, n = 16, T2 = 23.7, p<0.001; Nkt, n = 10, T2 = 64.1, p<0.001; flste370f/dt3Tpl, n = 6, T2 = 22.8, p<0.005). The dominant effect of Nkt and flsdt3Tpl seen in development of the scale pattern was not observed in the formation of skull size. However, an analysis of changes in the proportional development of the skull by measurements of the relative positioning of the eye within the skull (L1/L2, H1/H2; Panel A) showed a significant and dominant effect of Nkt, flsdt3Tpl on the patterning of the skull (Panel C). This effect was seen in flste370f homozygotes as well and was not specific to particular alleles of fls. The alteration in skull size and shape in the mutants does not involve loss of a particular organ structure or specific bone, rather a change in proportions of the developing skull. |
|
Scale formation and variation in the rs3/edar medaka mutant on the cs-2 background. (A) Alizarin-red stained rs3 medaka showed substantial scale formation and variation of the extent of scalation. (B) Wild type cs-2 strain scalation pattern. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|