- Title
-
Zebrafish curly up encodes a Pkd2 ortholog that restricts left-side-specific expression of southpaw
- Authors
- Schottenfeld, J., Sullivan-Brown, J., and Burdine, R.D.
- Source
- Full text @ Development
|
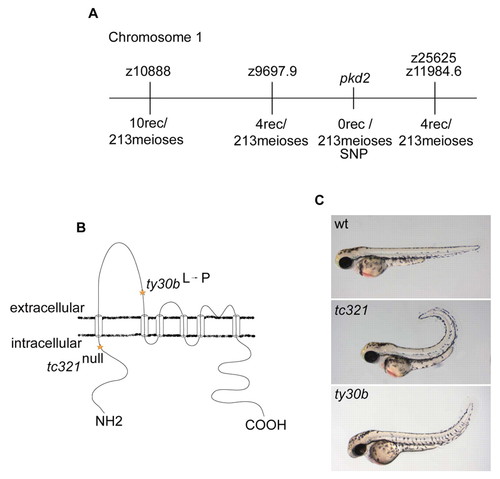
Identification and structural analysis of cup/pkd2. (A) Mapping of the cup mutation to a genetic interval on chromosome 1. The number of recombinants at each marker is shown. (B) pkd2 encodes a six-pass transmembrane calcium-activated non-specific cation channel [structure modified from Hayashi et al. (Hayashi et al., 1997)]. Sequencing of pkd2 in tc321 mutants revealed a nonsense mutation at nucleotide position 407 and ty30b showed a missense mutation at nucleotide position 1052, resulting in an amino acid substitution from a leucine to proline. (C) Lateral image of wild-type, cuptc321, and cupty30b embryos at 48 hpf. Both alleles have a curly tail upward phenotype, but ty30b has a milder curvature than that of tc321. PHENOTYPE:
|
|
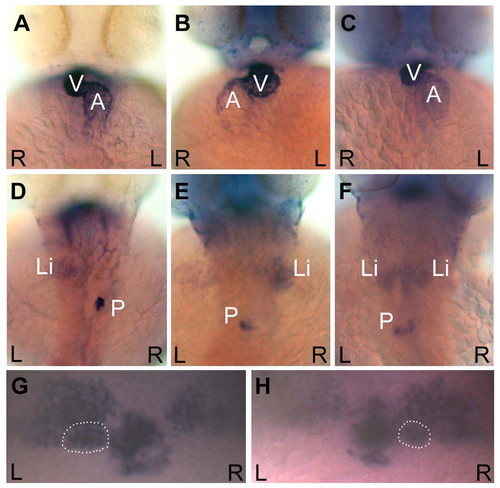
curly up embryos display defects in visceral and brain asymmetries. Ventral views of the heart (A-C) and dorsal views of the liver and pancreas (D-F) in cup embryos at 48 hpf. The heart, liver and pancreas were visualized by in situ hybridizations for cmlc2, fkd2, and ins, respectively. Wild-type organ positioning can be seen in approximately one third of a cup mutant population (A and D), where the ventricle loops to the right (A), and the liver resides on the left and the pancreas on the right (D). cup embryos also exhibit situs inversus, as seen by the complete reversal of the heart (B), liver and pancreas (E). Approximately one-third of cup mutants display heterotaxia. Included in this category was an embryo that had correct heart looping (C) but showed a duplicated liver with a reversed pancreas (F). (G,H) Dorsal views of the habenular nuclei and the pineal complex in 3-day cup embryos. The parapineal is outlined in each panel. (G) cup mutant with the wild-type pattern of more intense lov expression in the left habenula, and left parapineal placement visualized by otx5. (H) cup mutant with reversed diencephalic asymmetries. L, left; R, right; V, ventricle; A, atrium; Li, liver; P, pancreas. EXPRESSION / LABELING:
PHENOTYPE:
|
|
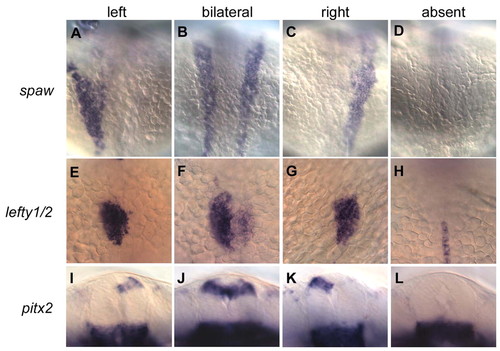
curly up affects expression of left-specific genes in the lateral plate mesoderm (LPM) and dorsal diencephalon. Dorsal views of spaw (A-D) and lefty1/2 (E-H) expression in 20- to 22-somite stage embryos (A-H) and ventral views of pitx2 expression in the dorsal diencephalon of 24-somite stage embryos (I-L) from cup heterozygote crosses. Alterations from normal left asymmetric Nodal signaling in the LPM (A) in cup mutants include bilateral (B), right (C), and absent (D) expression of spaw in this tissue. Over half of the bilaterally expressing embryos show only posterior propagation of spaw. Similarly, asymmetrically expressed downstream targets of Nodal signaling in the LPM and brain are also disrupted. lefty1/2, which are normally expressed in the left cardiac LPM (E), have bilateral (F), right (G), and absent (H) expression patterns in cup mutants. In H, midline expression of lefty1 can also be seen. pitx2, which is normally expressed on the left side of the diencephalon (I), can be expressed bilaterally (J), on the right (K), or not at all (L) in cup embryos. The majority of embryos show no expression of lefty1/2 in the LPM and diencephalon and no expression of pitx2 in the diencephalon. EXPRESSION / LABELING:
PHENOTYPE:
|
|
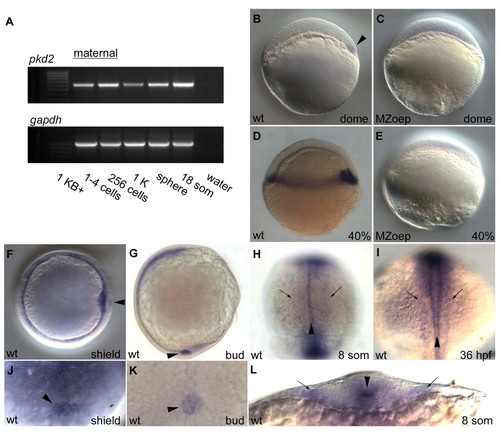
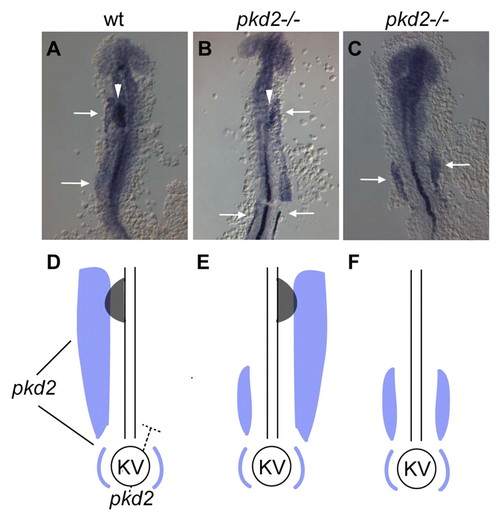
Expression analysis of pkd2. (A) RT-PCR of pkd2 at early developmental time-points shows presence of maternal pkd2 prior to mid-blastula transition at 1K stage. gapdh was used to control for variations in total RNA for each cDNA library. Maternal contribution of pkd2 RNA cannot be detected by in situ hybridization. (B-E) Lateral views of pkd2 expression patterns in wild-type (B,D) and MZoep (C,E) embryos; anterior is up. Expression of pkd2 is first noticeable at dome stage, where pkd2 expression marks the dorsal side of the embryo (arrowhead in B) and continues to be expressed in all dorsal marginal cells by 40% epiboly (D). pkd2 expression is in mesendodermal tissue as expression is lost in MZoep embryos (C,E). (F,J) pkd2 RNA is detected at the shield in gastrulating embryos, as well as in the dorsal forerunner cells (DFCs, arrowhead in F and J; F anterior view, dorsal to the right; J is a higher magnification dorsal view focusing on the DFCs). (G,K) At tailbud stage, pkd2 is detected in a circular patch of cells that will soon form Kupffer′s vesicle (arrowhead in G and K; G lateral view, anterior is up, dorsal to the left; K is a higher magnification posterior view of region in G denoted by the arrowhead). (H,I,L) Dorsal view of 8-somite (H) and 36-hpf (I) embryos and cross-section view of an 8-somite embryo (L). Faint pkd2 expression highlighting the pronephric collecting ducts is marked with arrows and the more intensely stained floorplate in the midline is marked with arrowheads. |
|
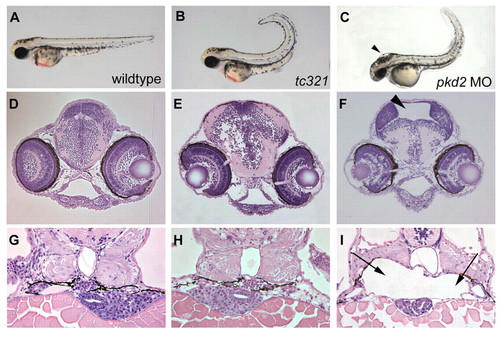
Morpholino knockdown of pkd2-/- causes hydrocephalus and dilations in the pronephric region in high doses. (A-C) Lateral views of 48-hpf embryos. Cross-sections of the brain (D-F) and kidney regions (G-I) at 72 hpf. The pkd2 augMO (4 ng) (C) and pkd2 SpMO (not shown) often have variably milder tail curls than that of a cuptc321 mutant (B) but noticeably different than that of a wild-type embryo (A). The embryos injected with either MO displayed hydrocephalus (F, SpMO) and dilations in the glomerular and tubular regions of the pronephros (I, SpMO) that are not observed in wild-type and cup embryos (D,E and G,H). Arrowheads indicate regions of hydrocephalus (C,F) and arrows indicate the dilations in the area of the pronephros (I). Note the smaller liver and gut tube underlying the pronephric dilations in MO-injected embryos (I). PHENOTYPE:
|
|
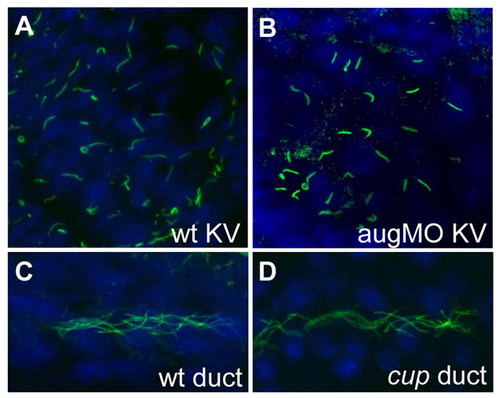
No obvious structural defects in cilia in Kupffer′s vesicle or pronephric ducts of pkd2 morphant and mutant embryos. (A-D) Immunofluorescence performed with antibody against acetylated tubulin that allows for visualization of cilia. Cilia at Kupffer′s vesicle in wild-type and pkd2 augMO embryos at 12 somites (A,B), and cilia in the pronephric ducts in wild type and in cuptc321 mutants (C,D), are similar in shape and length. Wild-type Kupffer′s vesicle cilia are 4.7±1.4 μm and pkd2 augMO Kupffer′s vesicle cilia are 4.6±1.3 μm in length. PHENOTYPE:
|
|
A model for altered asymmetric nodal gene expression in pkd2-deficient zebrafish embryos. (A-C) RNA in situ hybridization for spaw and lefty1/2 in 20-somite embryos that were flat-mounted in order to see all domains of expression. All panels show lefty1 staining in the midline. White arrows mark the anterior and posterior areas of spaw expression and white arrowheads mark lefty expression in the heart field. (D-F) Schematic interpretations of A-C (spaw in blue, lefty in gray). In a wild-type embryo (A,D), spaw is expressed in a posterior-to-anterior pattern on the LPM from 10 somites through 22 somites. lefty1/2 is activated in the left heartfield, the anterior portion of the LPM, beginning at 18 somites. RNA in situ hybridizations for spaw and lefty1/2 together in cup mutants (B,C) reveal a role for the anterior domain of spaw in the activation of the asymmetric expression of lefty1/2 in the cardiac LPM. In all embryos scored, lefty expression is only apparent in embryos where spaw reaches the anterior LPM and it always correlates with the side of spaw expression (A,B,D,E). cup mutants primarily exhibit bilateral expression of spaw to varying degrees of posterior to anterior extension (B,C,E,F). Bilateral anterior expression of spaw results in randomized lefty expression, where the heartfield will have left, right, bilateral, or absent lefty. Bilateral posterior expression of spaw always correlates with loss of lefty expression in the LPM (C,D). Therefore, because pkd2 zebrafish mutants display a defect in spaw propagation, lefty-expressing cells are largely absent from the LPM. In zebrafish, we propose that pkd2 is acting at Kupffer's vesicle (KV) to restrict spaw to the left side of the embryo and that pkd2 plays an additional role in the proper propagation of spaw anteriorly in the LPM (D). pkd2 could also restrict nodal to the left by acting at Kupffer's vesicle to repress nodal activation and propagation on the right LPM (D). EXPRESSION / LABELING:
|
|
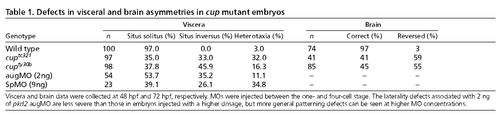
Defects in visceral and brain asymmetries in cup mutant embryos. PHENOTYPE:
|

Unillustrated author statements |