- Title
-
Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation
- Authors
- Wang, J., Hamblet, N.S., Mark, S., Dickinson, M.E., Brinkman, B.C., Segil, N., Fraser, S.E., Chen, P., Wallingford, J.B., and Wynshaw-Boris, A.
- Source
- Full text @ Development
|
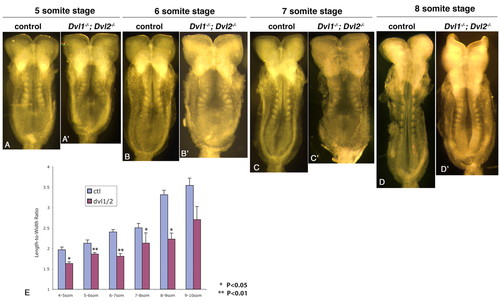
Dvl1-/-; Dvl2-/- mutants displayed reduced length-to-width ratio during neurulation. (A-D) Five- to eight-somite stage Dvl1-/-; Dvl2+/- or Dvl1-/-; Dvl2+/+ control embryos. (A'-D') Dvl1-/-; Dvl2-/- mutants of equivalent somite stages. Floor plates are expanded in Dvl1-/-; Dvl2-/- mutants. (E) Length-to-width ratio (LWR) changes of control and Dvl1-/-; Dvl2-/- mutant embryos at different somite stage during neurulation. Each point represents the average of at least three separate embryos. The error bars represent standard deviation. *P<0.05; **P<0.01. |
|
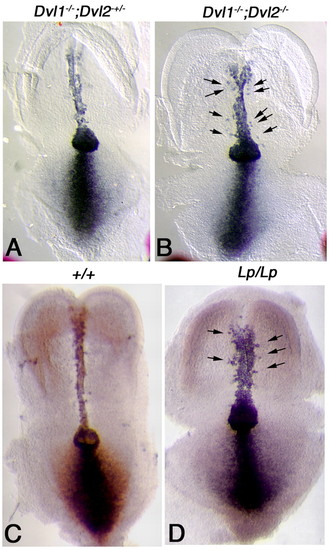
In situ hybridization analysis of Brachyury (T) in early headfold stage Dvl1-/-; Dvl2-/- and Lp/Lp mutant embryos. (A) T, a known downstream target gene of the canonical Wnt pathway, was expressed in the notochord, node and primitive streak in control embryos (Dvl1-/-; Dvl2+/-). (B) In Dvl1-/-; Dvl2-/- mutants, T expression in the node and primitive streak was maintained, suggesting normal activity of the Wnt pathway. However, T expression in the notochord was abnormally widened. T-positive cells were also observed outside of the notochord (arrows), consistent with a disruption of cell movement towards the central midline. Compared with wild-type littermates (C), T expression was also widened in the notochord of Lp/Lp embryos (D). T-positive cells were also observed outside of the notochord (D, arrows). |
|
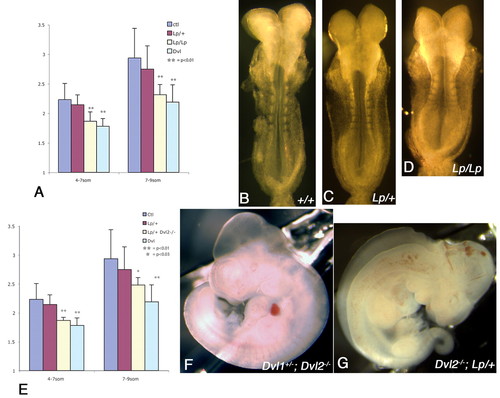
Genetic interaction between Dvl2 and Lp and reduced length-to-width ratio (LWR) in Lp/Lp and Dvl2-/-; Lp/+ embryos during neurulation. (A) Similar to Dvl1-/-; Dvl2-/- embryos, Lp/Lp mutants displayed significant reduction of LWR during neurulation, while Lp/+ embryos displayed intermediate reduction. At the eight-somite stage, some of the neural folds were already apposed and started to fuse at the dorsal midline in wild-type embryos (B). Parallel to the reduction of LWR, however, the neural folds were still open in Lp/+ embryos (C) and widely apart in Lp/Lp embryos (D) of the same somite stage. Similar to both Dvl1-/-; Dvl2-/- and Lp/Lp mutants, all Dvl2-/-; Lp/+ embryos also displayed significant reduction of LWR during neurulation (E). From a cross between Dvl1+/-; Dvl2+/-; Lp/+ and Dvl2-/- mice, all progeny that were Dvl2-/- or Dvl1+/-; Dvl2-/- (F) displayed normal neural tube closure at E10.5. By contrast, all Dvl1+/-; Dvl2-/-; Lp/+ and Dvl2-/-; Lp/+ embryos displayed craniorachischisis (G). The error bars represent s.d. |
|
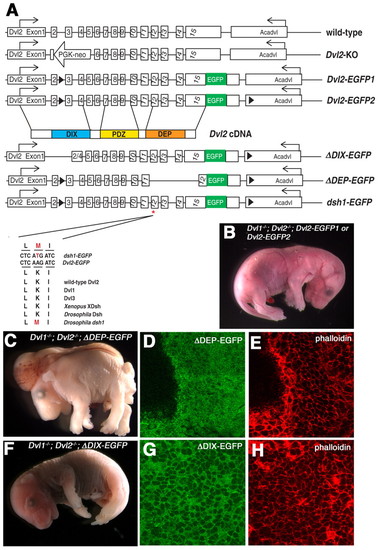
Domain requirement of Dvl2 for its involvement in convergent extension and membrane distribution during neurulation. (A) Schematic diagram of the genomic structure of the endogenous wild-type Dvl2 allele, the targeted Dvl2 null allele (Dvl2 KO), the two wild-type Dvl2 BAC transgenes, Dvl2-EGFP1 and Dvl2-EGFP2, and three mutant Dvl2 BAC transgenes: δDIX-EGFP, δDEP-EGFP and dsh1-EGFP. Although Dvl2-EGFP1 had an EGFP cassette fused in-frame to the last codon of Dvl2 and a LoxP site inserted into intron 2 for genotyping purpose (see Materials and methods), Dvl2-EGFP2 contained an additional LoxP site inserted in the 3' flanking gene Acadvl (acylcoenzyme A dehydrogenase, very long chain). δDIX-EGFP and δDEP-EGFP deletion mutant transgenes were generated by removing sequence encoding amino acids 67-159 and 442-736, respectively. dsh1-EGFP point mutation was generated by replacing AAG with ATG to introduce a K→M substitution at amino acid 446. Lower panel showed sequence alignment of the wild-type Dvl2-EGFP and the modified dsh1-EGFP BAC transgenes, as well as endogenous mouse Dvl1, Dvl2, Dvl3, Xenopus XDsh, Drosophila Dsh and the dsh1 mutation identified in fly. Although Dvl2-EGFP1, Dvl2-EGFP2 (B) and δDIX-EGFP (F) can fully rescue the neural tube closure defects in Dvl1-/-; Dvl2-/- mutants, δDEP-EGFP transgenes completely failed to rescue the neural tube defects in Dvl1-/-; Dvl2-/- mutants (C). Confocal analysis indicated that although δDIX-EGFP was primarily localized to the membrane (G,H), δDEP-EGFP was evenly distributed in the cytoplasm (D,E). |
|
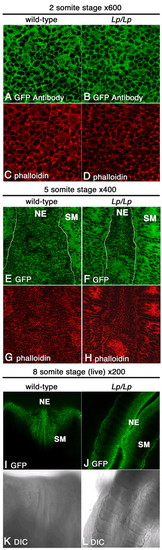
Dvl2-EGFP was primarily localized to the membrane in the neuroepithelium during neurulation. Single optical sections from laser-scanning confocal microscopy of neuroepithelium from neurulating embryos showed that, in the wild-type background, Dvl2-EGFP was primarily localized to the plasma membrane in the neuroepithelium (A,E,I). Consistent results were observed in wild-type and Lp/Lp mutant two-somite stage embryos stained with an anti-EGFP antibody (A,B) or through direct visualization of Dvl2-GFP signal from briefly fixed five-somite stage embryo (E,F) or live eight-somite stage embryos in culture (I,J). The apparently diffuse cytoplasmic distribution of Dvl2-EGFP and actin in some cells in A,B was due to scanning of the focal plane immediately underneath the apical plasma membrane during confocal laser microscopy. Wild-type and mutant embryos from the two- and five-somite stage were also stained with Phalloidin (C,D,G,H) to label F-actin. When the entire series of z stacks were examined (data not shown), it was clear that both Dvl and actin are localized to the lateral plasma membrane as individual optical sections are scanned more basally through the cell. Curiously, Dvl2-EGFP and actin distribution also appeared to be more diffuse in the somatic mesoderm at the five-somite stage (E-H). Overall Dvl2-EGFP distribution in the neuroepithelium was not altered in the Lp/Lp mutants (B,D,F,H,J,L; data not shown). (K,L) DIC images of live wild-type (K) or Lp/Lp mutant (L) embryos in culture. NE, neuroepithelium; SM, somatic mesoderm. |
|
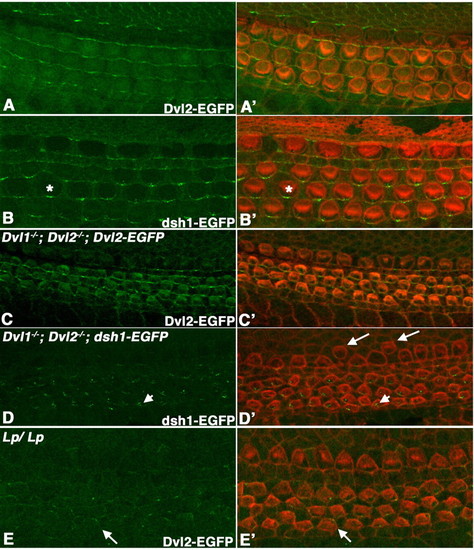
Localization of Dvl2-EGFP and dsh1-EGFP in the organ of Corti. (A-E) Confocal scanning at the apical surface of hair cells in whole-mount organ of Corti at E18.5, showing the native Dvl2-EGFP (A,C,E) or dsh1-EGFP (B,D) signal. (A'-E') are the overlay of Dvl2-GFP (green, A',C',E') or dsh1-EGFP (green, B',D') and hair cell membrane and stereociliary bundles (red) outlined with phalloidin staining. At E18.5 in an otherwise wild-type background, dsh1-EGFP localization along the distal membrane appeared to be more punctate (asterisk in B and B') when compared with wild-type Dvl2-EGFP (A,A'). By contrast, in Dvl1-/-; Dvl2-/- background at E16.5, dsh1-EGFP localization on the membrane was mostly lost and accumulation in the cytoplasm was observed (arrowheads in D,D'). Dvl1-/-; Dvl2-/-; dsh1-EGFP mutants also display mis-alignment of inner hair cells (arrows in D'). Although wild-type Dvl2-EGFP could maintain an even distribution along the distal membrane in Dvl1-/-; Dvl2-/- background at E16.5 (C,C'), its membrane localization was reduced and no longer restricted to the distal side (arrows in E,E') in Lp/Lp mutants at this stage. |
|
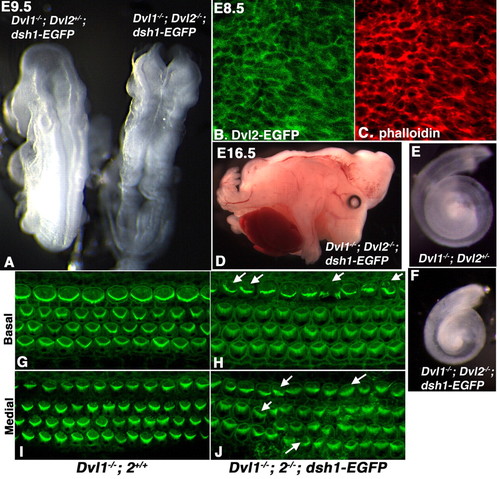
dsh1-EGFP failed to rescue the craniorachischisis, cochlear elongation and stereociliary polarity phenotypes in Dvl1-/-; Dvl2-/- embryos. (A,D) Dvl1-/-; Dvl2-/-; dsh1-EGFP embryos (right in A) displayed craniorachischisis at E9.5 and E16.5, suggesting that dsh1-EGFP was not able to compensate for the convergent extension defect in neurulation Dvl1-/-; Dvl2-/- mutants. (B) Confocal analysis indicated that dsh1-EGFP was primarily localized to the plasma membrane in the neuroepithelium. (C) Confocal analysis of phalloidin staining. (E,F) Cochlea recovered at p0 from Dvl1-/-; Dvl2+/- and Dvl1-/-; Dvl2-/-; dsh1-EGFP mice, indicating that dsh1-EGFP also failed to rescue the cochlea elongation defect in Dvl1-/-; Dvl2-/- mutants that was also thought to be due to disruption of convergent extension. (G,I) Confocal scan at either the base (G) or medial region (I) showing uniform stereociliary orientation in the organ of Corti of a p0 Dvl1-/-; Dvl2+/+ mouse. (H,J) However, in the organ of Corti of a Dvl1-/-; Dvl2-/-; dsh1-EGFP mouse, the stereociliary bundles were mis-oriented (arrows) in some hair cells. Misalignment in the outer hair cells could also be observed. |