- Title
-
Specification of the Primitive Myeloid Precursor Pool Requires Signaling through Alk8 in Zebrafish
- Authors
- Hogan, B.M., Layton, J.E., Pyati, U.J., Nutt, S.L., Hayman, J.W., Varma, S., Heath, J.K., Kimelman, D., and Lieschke, G.J.
- Source
- Full text @ Curr. Biol.
|
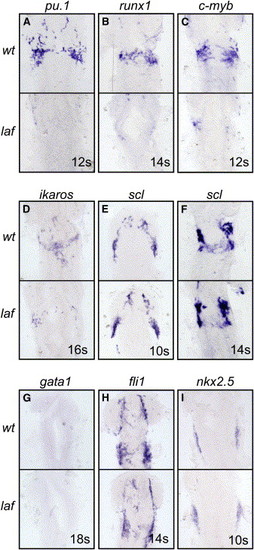
Alk8 Is Specifically Required for Myelopoiesis in the Anterior Lateral Plate Mesoderm (A–D) Gene expression marking cells of the anterior myeloid precursor pool was vastly reduced in lost-a-fin/alk8 (laf) mutant embryos. Expression of pu.1 (A) (n = 16), runx1 (B) (n = 13), c-myb (C) (n = 9), and ikaros (D) (n = 11) is shown. (E–I) scl expression, endothelial precursor, and myocardial precursor cell types derived from the ALPM develop normally in laf mutant embryos. Expression of scl (E, F) (n = 40 at 10 s and n = 46 at 14 s), gata1 (G) (n = 41), fli1 (H) (n = 9), and nkx2.5 (I) (n = 15) is shown. All images are dorsal views of the anterior of flat-mounted embryos, with the somitic age shown in the bottom right corner of each panel. The genotype was determined by examining the patterning phenotype of the embryos. EXPRESSION / LABELING:
|
|
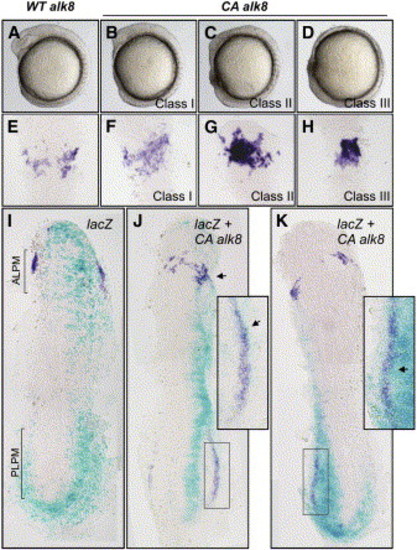
Alk8 Is Instructive in Myelopoiesis (A–H) Anterior expression of pu.1 was increased in ventralized embryos injected with constitutively active alk8 (CA alk8) mRNA. (A) Embryos injected with wild-type alk8 receptor mRNA (WT alk8) (10 ng/μL) are phenotypically wild-type. (B–D) Embryos injected with CA alk8 mRNA (10 ng/μL) are ventralized and were separated into three classes of increasing phenotypic severity: class I embryos display reduced anterior structures and small eyes (B); class II embryos have reduced anterior structures and have no eyes (C); and class III embryos have virtually no anterior structures and are severely ventralized (D) (all embryos at 8 somites). (E) Embryos injected with WT alk8 mRNA display normal expression of pu.1 at 14 hpf. (F) Class I embryos display mildly increased expression of pu.1 in the anterior (31%; n = 26). (G) Class II embryos display increased intensity and expansion of pu.1 expression in the anterior (85%; n = 46). (H) Class III embryos display a restricted region of pu.1 expression of increased intensity (94%; n = 18). (I–K) Increased expression of pu.1 is specific to the delivery of CA alk8 mRNA in mosaic embryos. (I) Embryos injected with lacZ mRNA only (50 ng/μL) into a single cell at the 1/16–1/32 cell stage display wild-type expression of pu.1 in lacZ-stained regions (n = 38 unilaterally lacZ-stained embryos displayed normal expression of pu.1). Embryo displayed at 8 somites. ALPM and PLPM are indicated by brackets. (J and K) Embryos coinjected with lacZ mRNA (50 ng/μL) and CA alk8 mRNA (40 ng/μL) display specifically increased pu.1 expression in regions of lacZ staining at 10 somites (J) and 8 somites (K) (n = 18/26 CA alk8, lacZ mosaics that stained unilaterally displayed coincidentally increased pu.1 expression). Arrowheads indicate increased pu.1; boxed sections in (J) and (K) are expanded to show close association of lacZ- and pu.1-positive cells. Embryos in (E)–(K) are flatmounted and viewed dorsally; (E)–(H) are dorsal views displaying ALPM at 12 somites; (I) and (K) are dorsal views of the whole embryo at 8 somites; and (J) is a dorsal view of the whole embryo at 10 somites. |
|
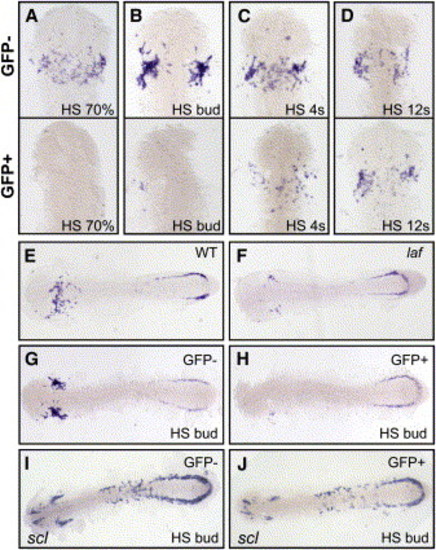
Blocking Embryonic Bmp Signaling Postpatterning of the Embryonic Axis Inhibits Myelopoiesis (A–D) Expression of pu.1 was lost in the anterior myeloid pool in embryos from a heat shock-inducible dominant-negative Bmp receptor transgenic line, after heat shock (HS) at 70% epiboly and bud stages. GFP-negative siblings (top) and GFP-positive siblings (bottom) (dorsal views displaying ALPM) were produced from a heterozygous outcross of the heat shock-inducible truncated Bmp receptor-GFP transgenic fish, all at 14–16 somites. (A) 70% epiboly heat shock (n = 63 GFP-negative embryos all expressed pu.1, n = 60/74 GFP-positive embryos displayed vastly reduced pu.1 expression). (B) Bud stage heat shock (n = 155 GFP-negative embryos all displayed pu.1 expression, n = 117/119 GFP-positive embryos displayed vastly reduced pu.1 expression). (C) 4 somites heat shock (n = 54 GFP-negative embryos displayed normal pu.1 expression, n = 45/77 GFP-positive embryos displayed only mildly reduced pu.1 expression). (D) 12 somites heat shock (n = 52 GFP-negative embryos all displayed pu.1 expression, n = 43 GFP-positive embryos all displayed normal pu.1 expression). (E–H) Transgenic animals heat shocked at bud stage phenocopy lost-a-fin expression of pu.1. In lost-a-fin mutants (F), anterior pu.1 expression is vastly reduced compared with wild-type (E), while posterior pu.1 shows no obvious reduction at 16 hpf. Likewise, in GFP-positive embryos from bud stage heat shocks (H) anterior pu.1 expression is vastly reduced compared to heat shocked GFP-negative siblings (G), while posterior pu.1 expression shows no obvious reduction at 14 hpf. Dorsal views of whole embryos. (I and J) Expression of scl is retained in the anterior and posterior in both GFP-negative ([I], n = 98) and GFP-positive ([J], n = 87) embryos after heat shock at bud stage. Dorsal views of whole embryos. |
|
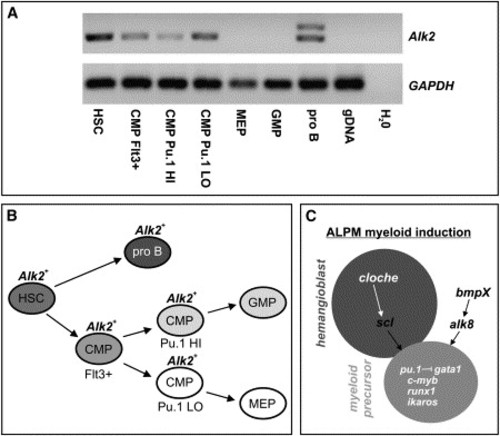
Expression of Alk2 in Murine Hematopoietic Precursors (A) Semiquantitative RT-PCR showing expression of Alk2 in cDNA from sorted hematopoietic precursors. Alk2 expression was detected in cDNA from hematopoietic stem cells (HSC), common myeloid progenitors (CMP) that were Flt3-positive, Pu.1-high expressing, and Pu.1-low expressing CMPs as well as in pro B cells (pro B) where two isoforms were detected. Expression was not observed in megakaryocyte-erythrocyte precursors (MEP) or in granulocyte-macrophage precursors (GMP). Primers flanked an intron and did not detect genomic DNA (gDNA). The Alk2 product is 403 bp and the GAPDH product 150 bp. (B) Diagrammatic representation of sorted cell types during hematopoietic development. HSCs give rise to pro B cells and CMPs, while Pu.1-high CMPs are thought to differentiate as GMPs and Pu.1-low CMPs are thought to differentiate as MEPs. Alk2 is expressed in all the early precursor populations but not in the more differentiated MEP or GMP cells. (C) Diagrammatic representation of the genetic pathways regulating the transition between the hemangioblast (dark gray) and myeloid precursor (light gray) cellular states in the zebrafish ALPM. Events occurring between bud and 4-somite stages are shown in black. |
|
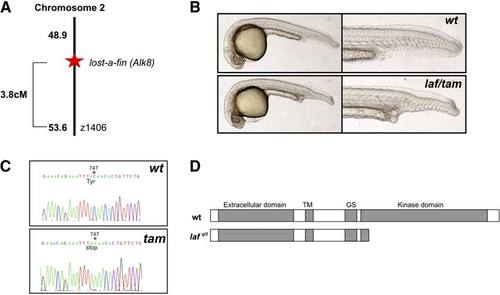
Molecular Characterization of the taminga Mutant taminga is allelic to the lost-a-fin locus. (A) SSLP mapping located the mutation on chromosome (LG) 2 linked to z1406, approximately 3.8 cM from the mutation and the losta- fin locus. (B) The myeloid failure mutant originally named taminga failed to complement lost-afin. Upper panels are wild-type siblings from a lost-a-fintm110b +/2 3 taminga +/2 (tam); Lower panels show a transheterozygote embryo that displayed the classical lost-a-fin phenotype (28%; n = 75). The mutant was hence designated laf gl2. (C) Sequencing wild-type and tam (laf gl2) mutant cDNA revealed a single base pair nonsense mutation at position 747 of the coding sequence. (D) Predicted outcome of the laf gl2 mutation is a carboxyl-truncated protein lacking the kinase domain, consistent with the functional null phenotype of the mutant previously described [S16, S17]. |
|
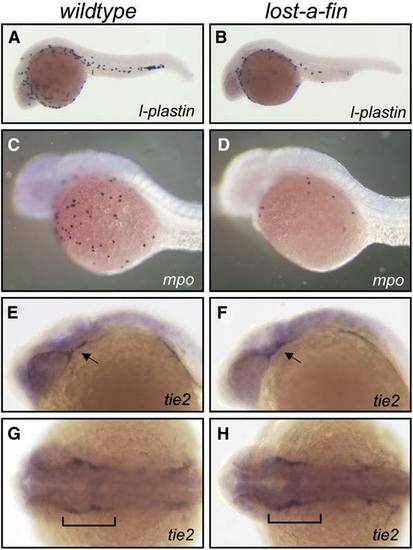
Expression of Markers of Myeloid and Endothelial Terminal Differentiation in lost-a-fin Mutants Markers of myeloid terminal differentiation were vastly reduced in lost-a-fin, but tie2 expression in differentiating vascular endothelial cells was normal. (A–D) The expression of l-plastin (24 hpf) (A) and myeloperoxidase (mpo) (30 hpf) (C) was vastly reduced in lost-a-fin mutants (B and D), mutant genotype was determined by the loss of the ventral tail fin. (E–H) The expression of tie2 (24 hpf) (E and G) was normal in lost-a-fin mutants (F and H), mutant genotype was determined by the loss of the ventral tail fin. All panels show lateral views except (G) and (H), which are dorsal views. Arrows in (E) and (F) and brackets in (G) and (H) indicate vascular staining. n > 30 progeny were analyzed in each experiment from crosses of known heterozygotes. |
|
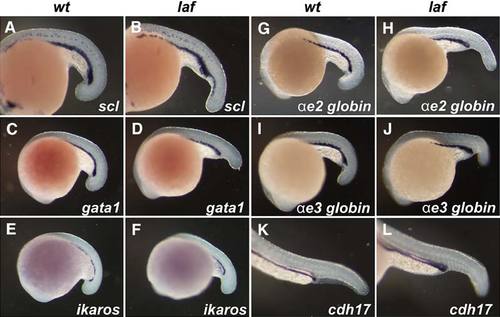
Posterior Hematopoiesis and Pronephric Duct Development Occurs Normally in lost-a-fin Mutants Normal developmental gene expression in the ventral hematopoietic tissues and pronephric duct was observed by in situ hybridization. The expression of scl (A, B), gata1 (C, D), ikaros (E, F), ae2 globin (G, H), and ae3 globin (I, J) at 18–20 hpf and of cadherin 17 (K, L) at 24 hpf in the ICM was unchanged in lost-a-fin mutant embryos; n > 30 progeny were analyzed in each experiment from crosses of known heterozygotes. EXPRESSION / LABELING:
|
|
Expression of gata1 and pu.1 in Class II CA alk8 mRNA-Injected Embryos Expression of gata1 and pu.1 was increased in the PLPM (as expected in ventralized embryos); pu.1 expression in the ALPM was expanded but gata1 was absent from the ALPM. gata1 expression was normal in WT alk8 mRNA-injected embryos (A) but was increased posteriorly in ventralized CA alk8 mRNA-injected embryos (B) (n = 37/54) at 8–10 somites. In embryos analyzed for expanded ALPM pu.1 expression (Figures 2E and 2G), posterior pu.1 expression was normal in WT alk8 mRNA-injected embryos with only weak expression posteriorly (C), but was increased in CA alk8 mRNA-injected embryos (D). (A) and (B) show gata1 expression, and (C) and (D) show pu.1 expression. |
|
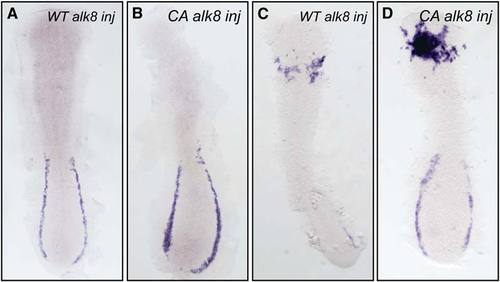
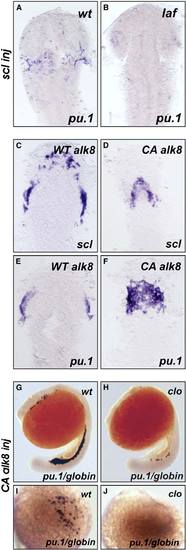
Genetic Epistatic Analysis of scl, cloche, and alk8 (A and B) lost-a-fin mutants injected with scl mRNA (50 ng/mL) did not display rescued expression of pu.1. Embryo in (A) is a wildtype sibling (n = 34) and in (B) a mutant sibling (n = 11). In the same experiment, scl mRNA injected from the same needle increased gata1 expression in posterior hematopoietic tissues (data not shown) as previously described [S18]. Embryo displayed as a flatmount, dorsal view of the anterior of the embryo. (C–F) Injection of CA alk8 mRNA does not increase scl expression. Embryos injected with WT alk8 mRNA (10 ng/mL) display normal expression of scl at 8 somites (C); embryos injected with CA alk8 mRNA (10 ng/mL) do not show an increase in scl expression at 8 somites (D) (n = 60/67 no increase [Class II], n = 7/67 vastly reduced anterior structures [Class III]). In the same experiment, CA alk8 mRNA-injected embryos displayed increased pu.1 expression (n = 62/105 increased) (F), but WT alk8 mRNA-injected embryos did not (E). Embryo displayed as a flatmount, dorsal view of the anterior of the embryo. (G–J) CA alk8 mRNA does not rescue pu.1 expression in cloche mutants. Wild-type siblings injected with CA alk8 mRNA, evidenced by mild ventralization, display normal expression of embryonic globin and pu.1 ([G], [I] [dorsal view], n = 46), whereas cloche mutants injected with CA alk8 mRNA, which were mildly ventralized, displayed vastly reduced globin expression and no expression of pu.1 (n = 24). Embryos in (G) and (H) are viewed laterally whereas (I) and (J) are views of the anterior. |