- Title
-
Notch1b and neuregulin are required for specification of central cardiac conduction tissue
- Authors
- Milan, D.J., Giokas, A.C., Serluca, F.C., Peterson, R.T., and MacRae, C.A.
- Source
- Full text @ Development
|
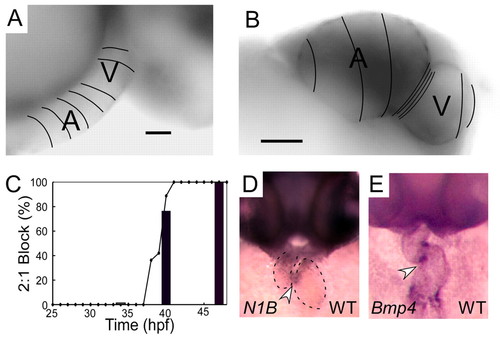
Normal development of AV conduction tissue. (A,B) Calcium activation maps from a single cardiac cycle in wild-type zebrafish at 36 hpf (A) and 48 hpf (B). Isochronal lines (50 mseconds) obtained by fluorescence microscopy are superimposed on maximum intensity projection images. These data demonstrate smooth conduction throughout the heart with ventricular acceleration at 36 hpf (A). Marked slowing at the AV junction can be seen at 48 hpf (B). Scale bars: 25 μm in A; 50 μm in B. (C) Time course of onset of 2:1 or higher grade AV block in response to atrial pacing (bars) or terfenadine (line) expressed as a percentage of the embryos studied. (D,E) In situ expression patterns of notch1b (D) and bmp4 (E) in wild-type embryos at 48 hpf. Arrowheads indicate expression in the AV ring a dotted line outlines the cardiac silhouette in D. EXPRESSION / LABELING:
|
|
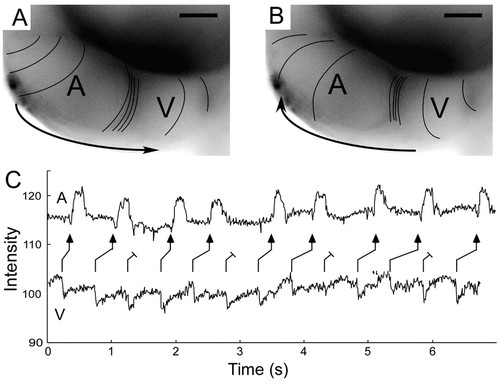
Characteristics of retrograde conduction in the zebrafish heart at 48 hpf. (A,B) Calcium activation maps for normal anterograde cardiac cycle (A) and spontaneous premature ventricular beat (B). Both anterograde and retrograde AV delay are evident as compressed spacing of isochronal lines (100 msecond intervals). Scale bars: 50 μm. (C) Contemporaneous recordings of atrial and ventricular contraction using intensity-time plots from regions over the respective chambers, during ventricular pacing at 48 hpf. The ladder diagram depicts the resulting Wenckebach-type retrograde ventriculo-atrial block. |
|
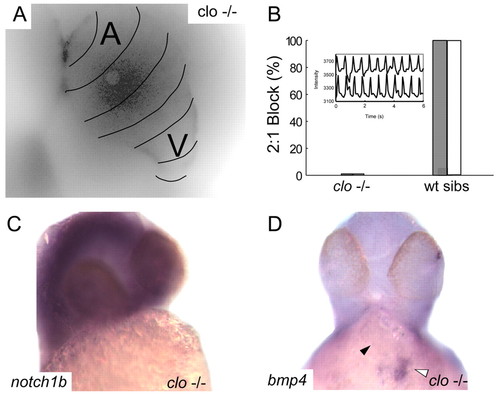
Endocardial signaling is required for the development of AV conduction tissue. (A) Calcium activation map of a single representative cardiac cycle in cloche mutant embryos at 48 hpf. Isochronal lines (black) obtained by fluorescence microscopy are superimposed on a maximum intensity projection of the same heart and demonstrate the failure of AV conduction tissue development in the absence of endocardium. (B) Lack of AV conduction block in cloche embryos compared with wild-type siblings is demonstrated by atrial pacing (white bars) and terfenadine exposure (gray bars). Inset of contemporaneous recordings of atrial and ventricular contraction using intensity-time plots from regions over the respective chambers in a terfenadine treated cloche embryo demonstrating 1:1 AV conduction. (C,D). In situ expression patterns of notch1b (C) and bmp4 (D) in cloche embryos at 48 hpf. Despite overstaining in the brain there is no evidence of notch1b signal in the heart (C). In D, the black arrowhead indicates the location of the AV ring; the white arrowhead denotes intense expression of bmp4 at the sinus venosus. |
|
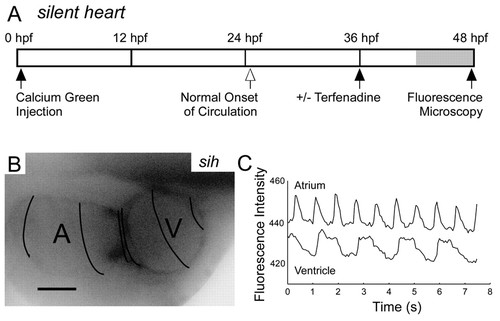
Flow is not required for the development of AV conduction tissue. (A) Time line of the experiments with silent heart embryos. (B) Calcium imaging of silent heart embryo at 48 hpf. Compressed spacing of isochronal lines (50 mseconds) in the AV ring demonstrates physiological conduction delay. Scale bar: 50 μm. (C) silent heart mutant embryos develop 2:1 block at 48 hpf in response to terfenadine treatment, as demonstrated in this plot of atrial and ventricular fluorescence intensity. |
|
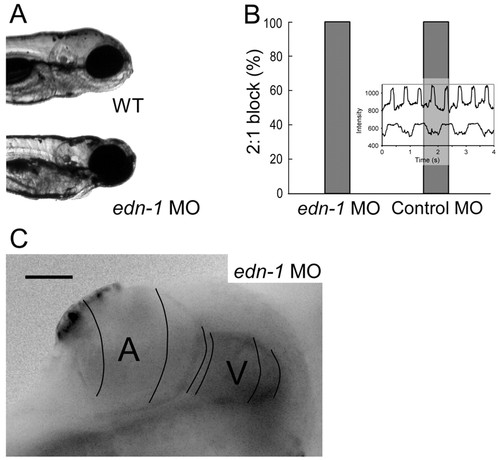
Endothelin 1 is not required for AV conduction tissue development. (A) Representative images of wild-type and endothelin 1 morphant fish. endothelin 1 morphants fail to develop structures derived from the pharyngeal arches, including the mandible. (B) endothelin 1 morphant fish develop normal AV conduction block on treatment with terfenadine at 48 hpf. Inset of contemporaneous recordings of atrial and ventricular contraction using intensity-time plots from regions over the respective chambers in an endothelin 1 morphant embryo demonstrating 1:1 AV conduction. (C) endothelin 1 morphant fish develop a normal AV conduction delay as seen in this calcium activation map of a single cardiac cycle at 48 hpf. Isochronal lines (50 mseconds) obtained by fluorescence microscopy are superimposed on a maximum intensity projection image. PHENOTYPE:
|
|
Neuregulin is necessary for the induction of AV conduction tissue. (A) Neuregulin is expressed widely in the developing brain (seen here at 48 hpf) and in the AV ring endocardium (arrowhead) from 36 hpf onwards. Inset shows a Hematoxylin and Eosin stained section of the AV canal in cross section with neuregulin staining (purple) in the AV ring endocardium. (B) neuregulin knockdown embryos fail to develop an AV conduction delay, as shown in this calcium activation map of a single cardiac cycle in a neuregulin morphant at 48 hpf. Isochronal lines (50 mseconds) obtained by fluorescence microscopy are superimposed on maximum intensity projection image. (C) neuregulin morphant embryos fail to develop an AV conduction block in the presence of terfenadine, as seen in contemporaneous recordings of atrial and ventricular contraction. (D) The morpholino effect is dose related, as seen in a histogram of the proportion of embryos that develop 2:1 AV block in the presence of terfenadine at 48 hpf. This proportion correlates with the amount of neuregulin mRNA detectable by RT-PCR from the respective embryos. |
|
Notch is required for the induction of AV conduction tissue. (A) notch1b knockdown embryos fail to develop an AV conduction delay, as shown in this calcium activation map of a single cardiac cycle in a notch1b morphant at 48 hpf. Isochronal lines (50 mseconds) obtained by fluorescence microscopy are superimposed on a maximum intensity projection image. (B) notch1b morphants fail to develop an AV conduction block on treatment with terfenadine, as seen in this histogram of the proportion of embryos developing 2:1 AV block at 48 hpf. Error bars indicate s.e.m. (C) neuregulin morphants exhibit normal expression of notch1b, as seen in this in situ hybridization. Arrowhead indicates cardiac expression in the AV ring. (D) notch morphants exhibit normal expression of neuregulin, as seen in this in situ hybridization. Arrowhead indicates cardiac expression in the AV ring. |
|
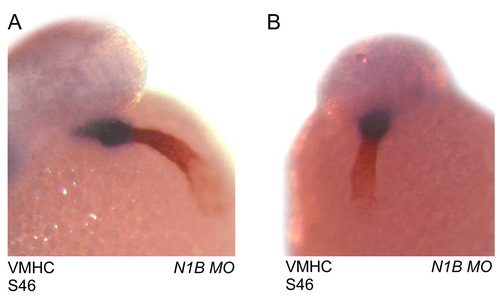
Atrial and ventricular specification is preserved in notch1b morpholino-treated zebrafish. (A,B) Front (A) and side (B) views of a notch1b morpholino-injected embryo stained with the S46 antibody (red) and a ventricular-specific myosin heavy chain (blue). EXPRESSION / LABELING:
|