- Title
-
Heart and soul/PRKCi and nagie oko/Mpp5 regulate myocardial coherence and remodeling during cardiac morphogenesis
- Authors
- Rohr, S., Bit-Avragim, N., and Abdelilah-Seyfried, S.
- Source
- Full text @ Development
|
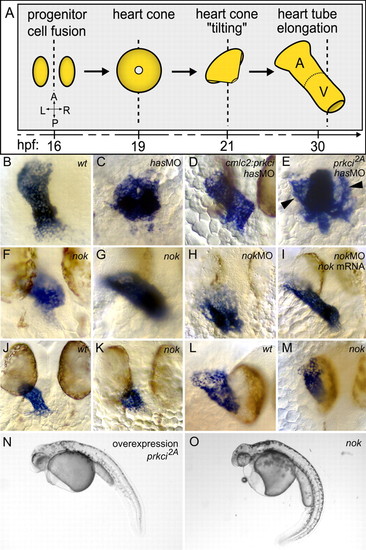
Cardiac morphogenesis in different genetic backgrounds. (A) Depiction of several key stages of zebrafish heart formation, beginning with the juxtaposition of two bilateral cardiac progenitor cell populations along the midline of the embryo (dotted line) to the elongation of the heart tube along the anterior-posterior axis. Dorsal view onto heart field. See text for detailed explanations. (B-I) cmlc2, (J,K) vmhc and (L,M) amhc expression. Embryos are 30-32 hpf. Parts L and M were captured with a 30° angle from dorsal for better visualization of atrial morphology. (B-D) Comparison of wild-type, has/prkci morphant and Tg(cmlc2:prkci) transgenic and has/prkci morphant embryos. (E) Heart cone formation is not completed, resulting in bilateral wings of myocardial progenitor cells (arrowheads) in prkci2A kinase-dead mutants. (F,G) Heart cone `tilting' occurs, but heart tube elongation is delayed to varying degrees in nok mutants. (F) Severe and (G) medium-strength phenotypes. (H) nok/mpp5 morphants display a severe heart tube elongation defect. (I) Co-injection of nok/mpp5 MO with wild-type nok/mpp5 mRNA rescues myocardial morphogenesis. There is no developmental delay. (J,K) Length of the ventricle is affected in nok mutants. (L,M) Length of the atrium is shortened and, along the mediolateral axis, narrower than wild type. (N,O) noks305 mutants and embryos that express high levels of PRKCi2A mutant protein (overexpression phenotype) are phenotypically similar. The body axis is curved ventrally and the retinal pigmented epithelium is largely disrupted, leaving the central portion of the retina uncovered, a phenotype more severe than in has mutants. EXPRESSION / LABELING:
|
|
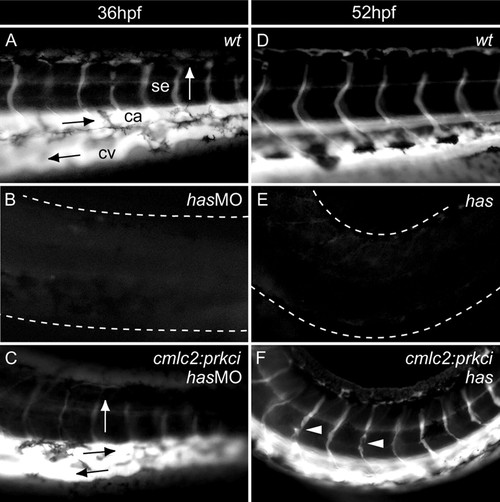
Has/PRKCi activity within myocardial cells is sufficient for heart function. Microangiograms depicting the peripheral circulation present within the tail region of embryos. The direction of blood flow is indicated by arrows. (A) wild-type embryo at 36 hpf. (B) has/prkci morphant at 36 hpf. (C) Tg(cmlc2:prkci) transgenic animal injected with the has/prkci MO at 36 hpf. (B) By contrast, peripheral circulation is never seen in has/prkci morphants. (D) Wild-type embryo at 52 hpf, with robust circulation. (E) Complete lack of peripheral circulation in a has mutant. (F) At 52 hpf, peripheral circulation is present in has mutants with Tg(cmlc2:prkci) insertion but defects in vessel formation can be observed (arrowheads). The white dotted line indicates the outline of the tail region. ca, caudal aorta; cv, caudal vein; se, segmental vessel. |
|
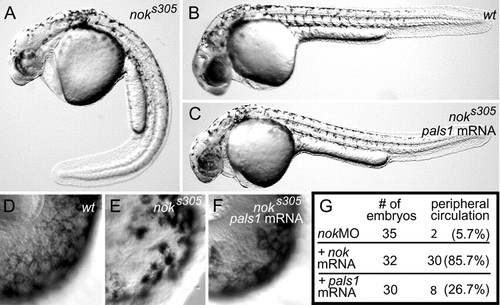
Nok is the functional homolog of mammalian Pals1/Mpp5. (A) noks305 mutant with ventrally curved body form and severely disrupted retinal pigmented epithelium at 34-36 hpf. (B) Wild type. (C) Murine Pals1/Mpp5 mRNA results in a partial phenotypic rescue of noks305 mutants, including the body form. (D) Wild-type retinal pigmented epithelium. (E) The noks305 retinal pigmented epithelium is severely disrupted. (F) Retinal pigmented epithelial cells are coherent in Pals1 mRNA-injected noks305 mutants (partial rescue). (G) A summary table showing the percentages of embryos with peripheral circulation among nok morphants injected with 50 ng/µl wild-type nok or 50 ng/µl Pals1/Mpp5 mRNA. |
|
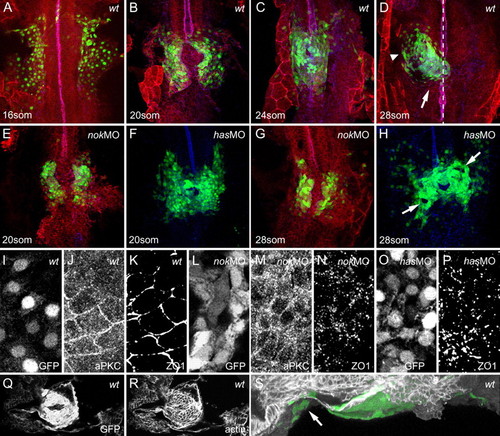
Myocardial epithelial organization is disrupted in has/prkci and nok/mpp5 morphants. All images represent reconstructions of confocal Z-stack sections imaged on whole mounts. (A-E,G) cmlc2:GFP, green (nuclear GFP within myocardial cells); PRKC/aPKC, red; ZO-1, blue. PRKC and ZO-1 were used as a counterstain to visualize the embryonic midline (see also dotted line in D). (F,H) cmlc2:GFP, green and ZO-1, blue. (Q-S) cmlc2:GFP and filamentous actin. (A) At the 16-somite stage, wild-type myocardial cells are organized into two bilateral sheets of cells. (B) Both sheets converge onto the midline, where they fuse to form the heart cone around the 20-somite stage. (C) Further convergence leads to a narrowing of the heart cone and expansion along the dorsoventral axis around the 24-somite stage. The entire heart cone moves out of the midline toward the left and anterior. (D) Heart cone tilting places the heart into the anterior-posterior orientation by the 28-somite stage. The atrium (arrowhead) is located to the left and anterior whereas the ventricle (arrow) is oriented toward the midline and posterior. (E,G) nok/mpp5 morphants display a delay in myocardial fusion to form the heart cone. (F,H) has/prkci morphants undergo heart cone fusion at the midline but tilting of the heart cone into the anterior-posterior orientation fails. Note the disrupted appearance of the myocardial layer, with holes and rough edges (arrows). (I-P) All images are details of confocal reconstructions of confocal Z-stacks imaged from 16-somite stage whole mounts, separated into the individual channels. (I-K) At the 16-somite stage, wild-type myocardial cells have an epithelial organization with junctional distribution of PRKC and ZO-1. (L-N) At the 16-somite stage, nok/mpp5 morphants display a diffuse distribution of PRKC along membranes and ZO-1 junctional belts are disrupted and appear as spots. (O,P) At the 16-somite stage, has/prkci morphants display a spotted distribution of ZO-1. (Q,R) Dorsal view onto the heart cone shown in S. (S) During tilting, specialized elongated myocardial cells containing actin-cables attach the heart cone to surrounding tissues (see arrow). Orientation: dorsal view and anterior to the top, except in S, lateral view and anterior to the left. EXPRESSION / LABELING:
|
|
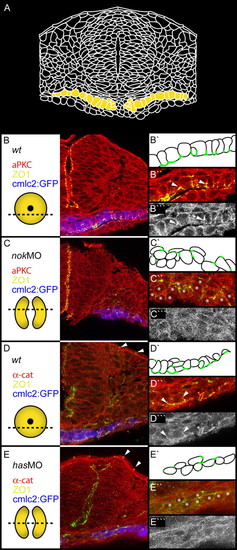
Apicobasal polarity is disrupted in has/prkci and nok/mpp5 morphants. Transverse sections of heart cone stage (20-somites) embryos. The section plane is indicated in each case (dotted lines). PRKC/aPKC and <α-catenin, red or gray; ZO-1, green; GFP false colored in blue. (A) Drawing of a transverse section through the center of the heart cone. Two bilateral wings of myocardial cells are indicated in yellow. (B,B'') In wild type, ZO-1 is localized to the apical membranes of the monolayered myocardial cell layer. (B''') PRKC is localized basal of ZO-1 (see arrowheads). (C,C'') nok morphants display diffusely positioned spots of ZO-1. (C''') PRKC is mislocalized in a diffuse pattern. (D,D'',D''') Co-localization of ZO-1 and α-catenin at the apical membrane in wild type. (E,E'',E''') In has morphants, co-localization of ZO-1 and ß-catenin is lost. The peridermal localization of ZO-1 and α-catenin is not affected (arrowheads). (E''') α-catenin is diffusely distributed along the membrane. Stars indicate myocardial cells. EXPRESSION / LABELING:
|
|
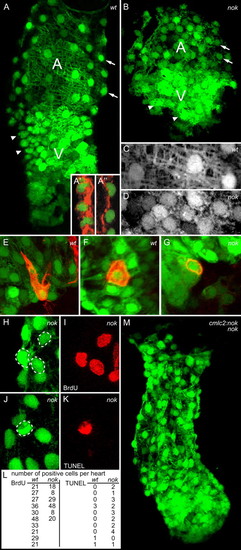
The zygotic function of nok/mpp5 is required for myocardial cell remodeling. (A,C,E,F) 36 hpf wild type and (B,D,G) noks305 in the Tg(cmlc2:GFP) transgenic background. (H-K) noks305 in the Tg(cmlc2:GFP) transgenic background at 32 hpf. Images are reconstructions of confocal Z-stack sections. (A,B) Arrows indicate atrial myocardial cells and arrowheads ventricular myocardial cells. Shape and density of noks305 mutant atrial and ventricular cells are similar. (A′,A′′) Merged confocal serial sections of actin (red) and nuclear stain (SYTOX green nuclear acid stain). (A′) Single sections and fusions of up to three sections of confocal serial stacks show that wild-type ventricular myocardial cells have cuboidal cell shapes. (A′′) wild-type atrial myocardial cells have squamous cell shapes. (C,D) Comparison of atrial myocardial cells in wild-type and noks305 mutant hearts, indicating that noks305 mutant atrial myocardial cells fail to expand in size and resemble ventricular myocardial cells. (E-G) Mosaic expression of mRFP within single Tg(cmlc2:GFP) transgenic myocardial cells. (E) Wild-type atrial myocardial cell. (F) Wild-type ventricular myocardial cell. (G) noks305 mutant myocardial cell. (H,I) BrdU analysis of noks305 mutant myocardial cells. White dotted lines indicate BrdU-positive cells. (J,K) TUNEL analysis of noks305 mutant myocardial cells. The white dotted line indicates a TUNEL-positive cell. (L) Quantification of BrdU- and TUNEL-positive cells. (M) nok/mpp5 functions tissue-autonomously within the myocardium during heart tube elongation. A noks305 mutant in the Tg(cmlc2:GFP) transgenic background that also carries the Tg(cmlc2:nok) rescue transgene displays an elongated heart tube similar to wild type. Orientation: dorsal view and anterior to the top. A, atrium; V, ventricle. |