- Title
-
Production of zebrafish germ-line chimeras from embryo cell cultures
- Authors
- Ma, C.G., Fan, L.C., Ganassin, R., Bols, N., and Collodi, P.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
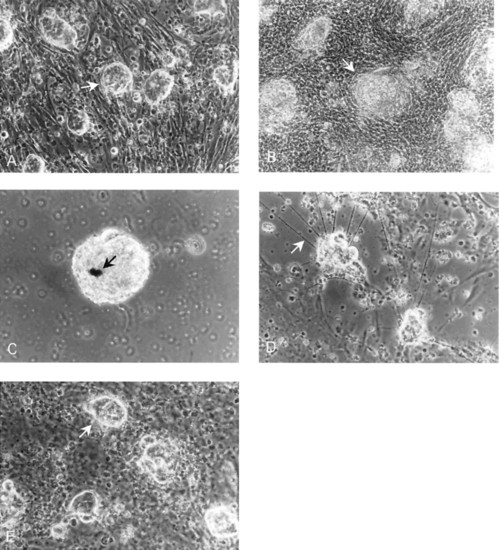
Phase contrast photomicrographs of zebrafish embryo cell cultures. (a) Culture (24 h) of embryo cell aggregates (arrow) on a feeder layer of RTS34st cells. (b) Culture (20 day old) of embryo cell aggregates (arrow) grown on an RTS34st feeder layer. (c) Culture (15 day old) maintained in the absence of feeder cells showing the presence of melanocytes (arrow). (d) Culture (10 day old) maintained in the absence of feeder cells showing the presence of neurites (arrow), indicating that neural cell differentiation has occurred. The neurites begin to appear in the culture on approximately day 5. (e) Culture (25 day old) of embryo cells in RTS34st cell-conditioned medium without a feeder layer, illustrating embryo cell aggregates (arrow) on a monolayer of embryo fibroblasts. |
|
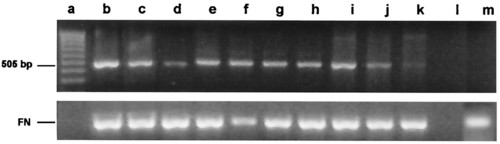
Reverse transcription-PCR analysis of vasa mRNA in zebrafish embryo cell cultures. cDNA was synthesized from total RNA obtained from embryo cell cultures. PCR amplification was performed with vasa-specific primers designed to generate a 505-bp product. Product identity was confirmed by sequencing. Lane a, MW markers; lanes b, c, and d, embryo cells maintained for 5, 15, and 25 days, respectively, in RTS34st cell-conditioned medium; lanes e, f, and g, embryo cells maintained for 5, 15, and 25 days, respectively, on an RTS34st feeder layer; lane h, embryo cells maintained at first for 24 days in RTS34st cell-conditioned medium, and then (after passaging) for 8 days on an RTS34st feeder layer; lanes i, j, and k, embryo cell cultures maintained for 1, 3, and 5 days, respectively, in the absence of RTS34st feeder cells or cell-conditioned medium; lane l, negative control (no template); lane m, RTS34st cells cultured in the absence of zebrafish embryo cells. Primers that amplify fibronectin cDNA were used to control for equal amounts of sample in each lane. |
|
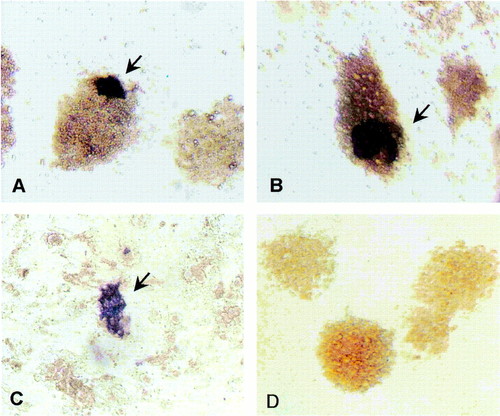
Distribution of vasa-positive embryo cells. Cultures maintained for 3 days (a) and 8 days (b) in RTS34st cell-conditioned medium or 3 days on RTS34st feeder cells (c) were examined by in situ hybridization by using a vasa-specific antisense probe. The control culture (d) was grown for 8 days in conditioned medium and hybridized with sense probe. [Magnification = x200 (a, b, and d), and = x100 (c).] |
|
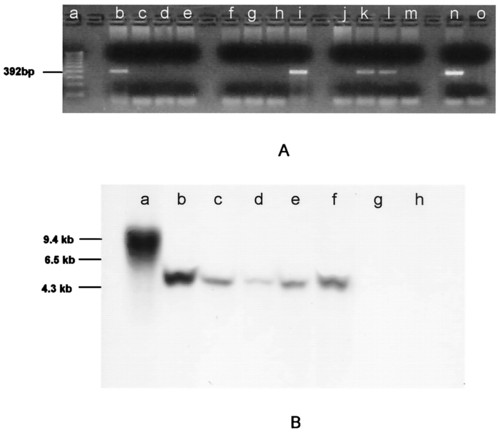
PCR (A) and Southern blot analysis (B) of genomic DNA showing the presence of neo sequences. (A) Genomic DNA isolated from individual F1 fish (lanes b-m) produced from a single spawning of GASSI fish that were injected as embryos with cultured cells and bred with noninjected GASSI individuals. DNA was amplified with neo-specific primers designed to generate a 392-bp product. Product identity was confirmed by sequencing. neo sequences were detected in lanes b, i, k, and l. Lanes a, n, and o are molecular weight markers, positive control (neo-containing plasmid template) and negative control (no template), respectively. (B) Southern blot analysis of genomic DNA by using a neo-specific probe. The same integration pattern for neo sequences was observed in DNA isolated from B7-43 fish (lane b), cell cultures derived from B7-43 embryos (lane c), individual F1 fish (lanes d and e) obtained from a GASSI chimera that was injected at the blastula stage with cultured B7-43 embryo cells and bred with a noninjected GASSI fish, and an F2 individual (lane f) obtained by breeding positive F1 siblings. Lane a, DNA isolated from fish embryo cells transfected in culture with neo-containing plasmid showing a different integration pattern. Lanes g and h, DNA isolated from a GASSI fish and another nontransgenic line of zebrafish. |
|
Zebrafish phenotypes. (a) A chimeric zebrafish from a GASSI embryo that had been injected at the blastula stage with cultured cells derived from B7-43 embryos. Melanocyte pigmentation is absent on the body of the chimera. (b) GASSI fish that was bred with the chimera shown in a to produce (c) F1 individuals that exhibited a pigmentation pattern characteristic of B7-43 and (d) the nonpigmented GASSI phenotype. |