Fig. 5
- ID
- ZDB-FIG-221220-57
- Publication
- Samper-Martín et al., 2021 - Polyphosphate degradation by Nudt3-Zn2+ mediates oxidative stress response
- Other Figures
- All Figure Page
- Back to All Figure Page
|
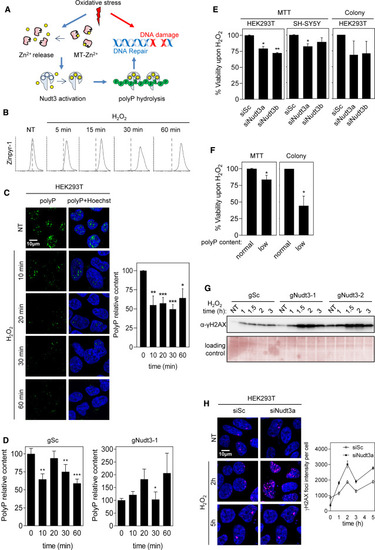
Figure 5. Nudt3 Zn2+-dependent polyP degradation is needed for cell viability upon oxidative stress (A) Working model. Oxidative stress leads to DNA damage and acts on the redox-sensitive metallothioneins (MTs) determining the release of intracellular Zn2+, which, in turn, activates the polyPase activity of Nudt3 and the depletion of polyP. The degradation of polyP protects DNA from oxidative damage and, as a result, cell viability is increased. (B) Oxidative stress increases intracellular free Zn2+ in HEK293T. Cells were exposed to 50 μM H2O2 at the indicated times, treated with Zinpyr for 1 h, and the green fluorescence intensity was measured by FACS analysis. 10,000 events were recorded. (C) Oxidative stress reduces polyP content. HEK293T cells were treated with 30 μM H2O2 for the noted time and immediately fixed. Polyphosphate content was detected, and representative pictures are in the left panel. The quantification of three independent experiments is in the right panel. The mean ± SEM is provided. A minimum of 400 cells per condition were analyzed. (D) PolyP reduction upon oxidative stress is Nudt3 dependent. HEK293T cells, in which Nudt3 has been depleted by CRISPR-Cas, were treated with 30 μM H2O2 for the noted times and immediately fixed. Scrambled guide (gSc) is used as control and gNudt3-1 is the specific guide for targeting Nudt3 gen. Polyphosphate content was detected by immunofluorescence as in Figure 3A. The graph represents the quantification of three independent experiments. The mean ± SEM is provided. A minimum of 400 cells per condition were analyzed. (E and F) Cell viability upon oxidative stress is dependent on Nudt3 polyPase activity. (E) Nudt3 was silenced in HEK293T and SH-SY5Y. After 48 h, 30 μM H2O2 was added, and 24 h later, MTT was performed. In the case of colony formation assays, cells were treated with 100 μM H2O2 for 1 h. Mean ± SEM from three independent experiments is shown. (F) Nudt3 was overexpressed as in Figure 3C (note that overproduction of Nudt3 leads to polyP depletion, noted as low polyP content. This effect is similar to being unable to use polyP as a result of Nudt3 silencing). Viability was measured as MTT and colony-forming in HEK293T cells were grown in the absence or presence of 30 μM H2O2 for 24 h (for the MTT experiments) and 100 μM for 1 h for the colony-forming assays. Mean ± SEM from three independent experiments is represented. (G and H) DNA damage upon oxidative stress is Nudt3 dependent. (G) Nudt3 was knocked down in HEK293T cells by using two different CRISPR-Cas9 guides. After 72 h, cells were treated with 100 μM H2O2 for 1 h and washed with fresh medium. Histone H2AX phosphorylation was detected by western blot with specific antibodies. Time is from the moment in which H2O2 was added. (H) Nudt3 was silenced in HEK293T cells. After 48 h cells were treated with 100 μM H2O2 for 1 h and washed with fresh medium. Histone phosphorylated foci were detected by immunofluorescence (using specific antibodies as in G). Representative images and fluorescence intensity (mean ± SEM) quantification from one experiment where a minimum of 250 cells were analyzed. The scale bars represent 10 μm. See also Figures S3 and S4. ∗∗∗p < 0.001; ∗∗p < 0.01; ∗p < 0.05. |