Fig. 2
- ID
- ZDB-FIG-170607-13
- Publication
- Kelu et al., 2017 - Ca2+ release via two-pore channel type 2 (TPC2) is required for slow muscle cell myofibrillogenesis and myotomal patterning in intact zebrafish embryos.
- Other Figures
- All Figure Page
- Back to All Figure Page
|
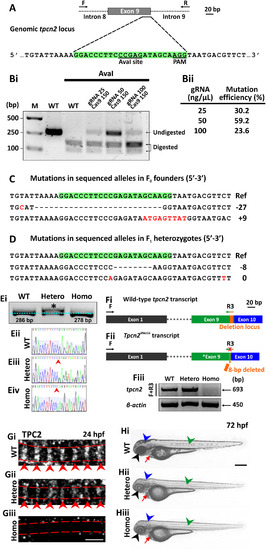
CRISPR/Cas9-induced mutagenesis of tpcn2. (A) Design of the 23-bp tpcn2 target sequence. (Bi) Evaluation of the mutation efficiency (with/without AvaI digestion) in the F0 injected embryos as shown by 2% agarose gel electrophoresis. The incomplete digestion observed might be due to the presence of a mutation within the restriction site. M is the DNA marker. (Bii) The mutation efficiency in embryos injected with different concentrations of gRNA; this was calculated by dividing the band intensity of the undigested band by the intensity of the undigested and digested bands. (C,D) Sanger sequence analysis of (C) two representative mutant alleles found in the F0 founder embryos and of (D) two separate germline transmitted mutant alleles, which were identified in two F1 heterozygous fish after the F0 founder fish were outcrossed. Ref is the reference sequence. (Ei) Representative 3% agarose gel electrophoresis showing the genotype of the tpcn2dhkz1a mutant. In the heterozygote, there are two bands; this is due to the formation of a DNA heteroduplex, comprising the WT and mutant DNA, with the latter migrating more slowly. (Eii-Eiv) This was confirmed by subsequent Sanger sequence analysis of the corresponding DNA samples. The red arrowheads indicate the location of the 8-bp deletion. (Fi) The wild-type tpcn2 mRNA transcript (showing just exons 1, 9 and 10) and the design of the forward (F) and reverse (R3) primers used for RT-PCR. The target sequence of R3 spans the deletion locus in the tpcn2dhkz1a mutant transcript (see orange rectangle in exon 9). (Fii) The tpcn2dhkz1a mutant mRNA transcript (showing just exon 1, the mutated exon 9, and exon 10), and showing the location of the 8-bp deletion (see orange arrow in exon 9). (Fiii) Representative 2% agarose gel electrophoresis showing the absence of the RT-PCR product in the tpcn2dhkz1a homozygote using the F+R3 primer set. β-actin was used as the internal control. (Gi-Giii) Representative confocal single optical sections of SMCs in (Gi) wild-type and (Gii-Giii) tpcn2dhkz1a mutant embryos at 24 hpf, after they were immunolabeled with the anti-TPC2 antibody, to show the down-regulation and disappearance of TPC2 striations, respectively. The red dashed lines indicate the SMC boundaries, which were revealed by phalloidin-labeling (data not shown); whereas the red arrowheads indicate the TPC2 banding pattern of localization. (Hi-Hiii) Representative bright-field images of: (Hi) wild-type, and (Hii-Hiii) tpcn2dhkz1a mutant embryos at 72 hpf to show the reduction in pigmentation in the eye, head, and trunk of the mutant larvae (see black, blue, and green arrowheads, respectively). An accumulation of blood cells was also seen in the heart of the mutants (see red arrows). In panels (E-H), WT, Hetero, and Homo are wild-type, heterozygotes and homozygotes, respectively. Scale bars, 3 µm in panel (G); and 250 µm in panel (H). |
| Fish: | |
|---|---|
| Observed In: | |
| Stage: | Protruding-mouth |
Reprinted from Developmental Biology, 425(2), Kelu, J.J., Webb, S.E., Parrington, J., Galione, A., Miller, A.L., Ca2+ release via two-pore channel type 2 (TPC2) is required for slow muscle cell myofibrillogenesis and myotomal patterning in intact zebrafish embryos., 109-129, Copyright (2017) with permission from Elsevier. Full text @ Dev. Biol.