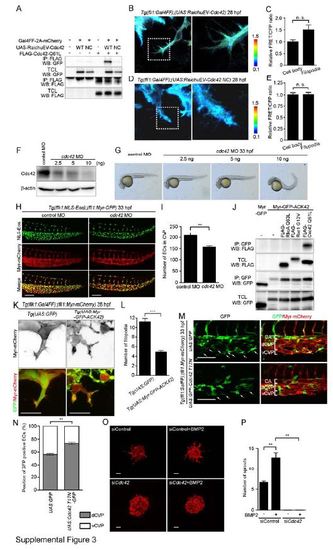
Cdc42 Is Required for CVP Formation. Related to Figure 3. (A) 293T cells were transfected without or with Gal4-driven expression vector for RaichuEV-Cdc42 (UAS:RaichuEV-Cdc42 WT) or its negative control (UAS:RaichuEV-Cdc42 NC) together without (-) or with (+) the plasmid encoding Gal4FF-2A-mCherry. Expression plasmid encoding FLAG-tagged constitutive active mutant of Cdc42 (FLAG-Cdc42 Q61L) was also transfected as indicated at the top. Immunoprecipitates (IP: FLAG) of cell lysates and aliquots of total cell lysate (TCL) were subjected to Western blot analyses with anti-GFP and anti-FLAG antibodies as indicated on the left. Note that active form of Cdc42 bound to RaichuEV-Cdc42, but not RaichuEV-Cdc42 NC. (B) 3D-rendered confocal images of the EC in the CVP of 28 hpf Tg(fli1:Gal4FF);(UAS:RahicuEV-Cdc42) embryo that expresses a FRET-based biosensor for Cdc42 in the ECs. The ratio images of FRET/CFP are shown in the IMD mode. The upper and lower limits of the ratio range are indicated on the right. The boxed area is enlarged on the right side. (C) Relative FRET/CFP ratio (Cdc42 activity) in the filopodia and cell body of EC in the CVP as observed in B was quantified and expressed as fold increase relative to that observed in the cell body. Data are shown as mean ± s.e.m. (n=11). Note that the FRET/CFP ratio in the filopodia was significantly higher than that in the cell body, suggesting that Cdc42 activity is increased in the filopodia extending from the ECs during CVP formation. (D) 3D-rendered confocal images of EC in the CVP of 28 hpf Tg(fli1:Gal4FF);(UAS:RahicuEV-Cdc42 NC) embryo that expresses a negative control FRET biosensor in the ECs are shown, as in B. (E) Relative FRET/CFP ratio in the filopodia and cell body of EC in the CVP as observed in D was quantified similar to C (n=5). Note that the FRET/CFP ratio in the filopodia was not different from that in the cell body, supporting high Cdc42 activity in the filopodia. (F) Lysates from 48 hpf zebrafish embryos injected either with 5 ng control MO or with 2.5 ng, 5 ng or 10 ng cdc42 MO were subjected to Western blot analysis with the anti-Cdc42 antibody and anti-β-actin antibodies. (G) Lateral views of 33 hpf embryos injected either with 5 ng control MO or with 2.5 ng, 5 ng or 10 ng cdc42 MO. (H) Confocal z-stack images of the caudal regions of 33 hpf Tg(flk1:NLS-Eos);(fli1:Myr-mCherry) embryos injected with 5 ng control MO or 5 ng cdc42 MO. Eos (NLS-Eos) and mCherry (Myr-mCherry) images and the merged images are shown as indicated at the left. (I) The number of ECs in the CVP as observed in H was counted, and shown as mean ± s.e.m. (control MO [n=6], cdc42 MO [n=6]). (J) 293T cells were transfected with the plasmid encoding either Myr-GFP or Myr-GFP-ACK42 together without (-) or with the vector expressing FLAG-tagged constitutive active mutant of RhoA (FLAG-RhoA Q63L), Rac1 (FLAG-Rac1 G12V) or Cdc42 (FLAG-Cdc42 Q61L) as indicated at the top. Immunoprecipitates (IP: GFP) of cell lysates and aliquots of total cell lysate (TCL) were subjected to Western blot analyses with anti-GFP and anti-FLAG antibodies as indicated on the left. Note that Myr-GFP-ACK42 specifically associated with active form of Cdc42, but not with those of RhoA and Rac1. (K) Projection view of confocal z-stack images of the CVP in 29 hpf Tg(UAS:GFP) (left panel) or Tg(UAS:Myr-GFP-ACK42) (right panel) embryos of Tg(flk1:Gal4FF-2A-mCherry);(fli1:Myr-mCherry) background. mCherry images and the merged images of (GFP [green]; Myr-mCherry [red]) are shown. (L) Filopodia number of each EC located at the vascular front as observed in K was quantified, as in Figure 1D (GFP [n=20], Myr-GFP-ACK42 [n=20]). (M) Projection view of confocal z-stack images of the caudal regions of 33 hpf Tg(fli1:Gal4FF);(fli1:Myr-mCherry) embryos injected with UAS:GFP Tol2 plasmid or UAS:GFP-Cdc42 T17N Tol2 plasmid. GFP images and the merged images of GFP (green) and mCherry (red) are shown at the left and right panels, respectively. Arrows indicate GFP-expressing or GFP-Cdc42 T17N-expressing ECs that localize in the ventral part of CVP. CA, caudal artery; dCVP, dorsal part of CVP; vCVP, ventral part of CVP. (N) Percentages of GFP-expressing or GFP-Cdc42 T17N-expressing ECs that localize in dCVP or vCVP, as observed in M, are shown as means ± s.e.m. (GFP [n=7], GFP-Cdc42 T17N [n=8]). (O) Projection view of confocal z-stack images of the spheroids formed by HUVECs transfected with control siRNA (siControl) or Cdc42 siRNA (siCdc42), and stimulated without or with BMP2. The spheroids were visualized by staining with rhodamine-phalloidin (F-actin). (P) The number of sprouts extended from each spheroid as observed in O was quantified (siControl [n=3], siControl + BMP2 [n=3], siCdc42 [n=3], siCdc42 + BMP2 [n=3]). Scale bars, 50 µm (H), 30 µm (K), 100 µm (M) and 60 µm (O). **p<0.01, ***p<0.001.
|

