Fig. S6
|
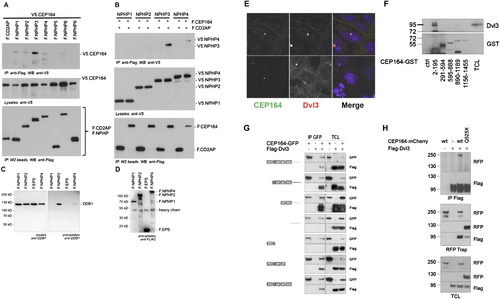
Molecular Interaction of NPHP-RC Gene Products with DDR Proteins. (A and B) Interaction of NPHP3 with CEP164. (A) To determine whether CEP164 interacts with known NPHP proteins, HEK293T cells were cotransfected with expression constructs for N terminally V5-tagged human full-length CEP164 and FLAG-tagged human full length NPHP1-NPHP5, NPHP8, NPHP9, or the control protein CD2AP as indicated. Comparable amounts of CEP164 were present in all lysates (middle panel). NPHP proteins were precipitated, using anti-Flag M2 beads (bottom panel). NPHP3 immobilized V5-tagged CEP164; a weak interaction was also detectable for NPHP1, 2 and 4 (top panel). (B) The reciprocal interaction confirmed an interaction between CEP164 and NPHP3. Flag-tagged CEP164 or CD2AP were coexpressed with V5-tagged NPHP1-NPHP4. Precipitation of NPHP3, but not the control protein CD2AP immobilized V5-tagged NPHP3. A weak, but reproducible interaction was also detected for NPHP4 (top panel). (C and D) Interaction of NPHP2 with DDB1. (C) HEK293T cells were transiently transfected with the Flag-tagged NPH-proteins NPHP1, NPHP2, NPHP4 or a control protein (Flag-EPS). Following immunoprecipitation with Flag-antibody Western blot analysis revealed that endogenous DDB1 coprecipitated with NPHP2 and to a lesser extent with NPHP4 but not with NPHP1 or the control protein. (D) Control experiment for protein expression using anti-FLAG antibody. (E and H) Cep164 and Dvl3 are in a precipitable complex. (E) CEP164 and Dvl colocalize at centrosomes. Whereas CEP164 is known to localize to the mother centriole only, Dvl3 is noted to label both centrioles (upper panel) or to the daughter centriole only (lower panel). HEK293 cells were fixed, labeled with antibody α-CEP164-PR and α-Dvl3. (F) Domain mapping of the CEP164 interaction with Dvl3. Series of bacterially produced CEP164-GST fragments were incubated with HEK293 cell lysates, precipitated using GST pull-down, and probed for presence of Dvl3 by immunoblotting against Dvl3 and GST, respectively. Endogenous Dvl3 interacts with a region within the first 194 amino acids of CEP164, which corresponds to the ATRIP binding domain. (G) CEP164 interacts with the proline-rich region of Dvl3. HEK293 cells were transfected with GFP-tagged full-length human CEP164 and a panel of truncation mutants of Flag-Dvl3 as indicated, and coimmunoprecipitation was performed with an anti-GFP antibody. Note that CEP164 only interacts with Dvl3 fragments that contain the proline-rich region (Pro). (H) Full length CEP164 but not the mutant CEP164-Q525X interacts with Dvl3. HEK293 cells were transfected with the indicated plasmids and subjected to immunoprecipitation using antibodies against FLAG-Dvl3 (upper panel) or CEP164-mCherryRFP (middle panel). Immunoblot analysis demonstrates strong interaction with Dvl3 for wild-type (wt) CEP164-mCherryRFP, whereas interactions is strongly reduced for CEP164-Q525X to Dvl3. |
Reprinted from Cell, 150(3), Chaki, M., Airik, R., Ghosh, A.K., Giles, R.H., Chen, R., Slaats, G.G., Wang, H., Hurd, T.W., Zhou, W., Cluckey, A., Gee, H.Y., Ramaswami, G., Hong, C.J., Hamilton, B.A., Cervenka, I., Ganji, R.S., Bryja, V., Arts, H.H., van Reeuwijk, J., Oud, M.M., Letteboer, S.J., Roepman, R., Husson, H., Ibraghimov-Beskrovnaya, O., Yasunaga, T., Walz, G., Eley, L., Sayer, J.A., Schermer, B., Liebau, M.C., Benzing, T., Le Corre, S., Drummond, I., Janssen, S., Allen, S.J., Natarajan, S., O'Toole, J.F., Attanasio, M., Saunier, S., Antignac, C., Koenekoop, R.K., Ren, H., Lopez, I., Nayir, A., Stoetzel, C., Dollfus, H., Massoudi, R., Gleeson, J.G., Andreoli, S.P., Doherty, D.G., Lindstrad, A., Golzio, C., Katsanis, N., Pape, L., Abboud, E.B., Al-Rajhi, A.A., Lewis, R.A., Omran, H., Lee, E.Y., Wang, S., Sekiguchi, J.M., Saunders, R., Johnson, C.A., Garner, E., Vanselow, K., Andersen, J.S., Shlomai, J., Nurnberg, G., Nurnberg, P., Levy, S., Smogorzewska, A., Otto, E.A., and Hildebrandt, F., Exome Capture Reveals ZNF423 and CEP164 Mutations, Linking Renal Ciliopathies to DNA Damage Response Signaling, 533-548, Copyright (2012) with permission from Elsevier. Full text @ Cell