Fig. 5
|
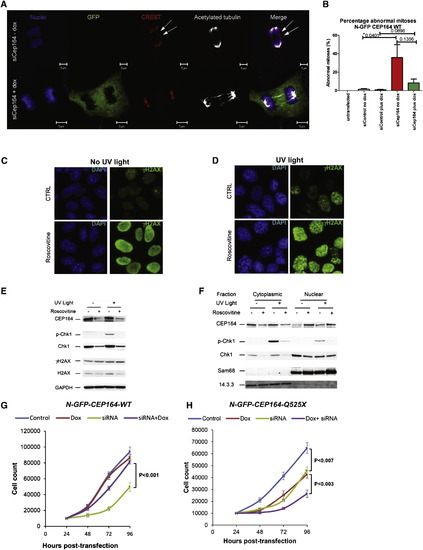
Knockdown of Cep164 Causes Anaphase Lag and Retarded Cell Growth (A and B) Knockdown of CEP164 causes anaphase lag. siCep164 knockdown in IMCD3 cells increased anaphase lag incidence from 1% after siCtrl to 21% after siCep164-treated cells (n > 250 anaphases, five independent experiments). CREST antiserum (red) and DAPI (blue) confirmed the presence of incomplete mitotic congression and unattached kinetochores during late anaphase (white arrows). Doxycycline-inducible expression of WT-CEP164 during Cep164 siRNA knockdown reduced the incidence of anaphase lag to 4%, whereas untransfected IMCD3 cells had no detectable anaphase lag (0%) (B). Bars represent SEM, p values (Student’s t test) are indicated above the bar graph. (C–F) The effect of roscovitine on UV-induced DDR. Cells were UV irradiated with 30 J/m2 and analyzed 1 hr after UV irradiation. Where indicated, cells were preincubated for 24 hr with the CDK inhibitor roscovitine (80 μM). (C and D) Immunofluorescence analysis showed that roscovitine triggered uniform nuclear distribution of γH2AX (activated H2AX phosphorylated at Ser139) in non-UV irradiated cells suggesting partial DDR activation (C). UV radiation caused enhanced γH2AX staining with a prominent nuclear foci pattern, characteristic of strong DDR activation (D). (C and D) Error bars denote SEM. (E and F) The effect of roscovitine on UV-triggered subcellular localization of CEP164 and Chk1. CEP164 and Chk1 proteins, along with nuclear marker Sam68 and cytoplasmic marker 14.3.3 were analyzed by Western blot. Roscovitine decreased the amount of CEP164 present in control and UV-irradiated cells (E). This was most likely due to translocation of CEP164 into the nucleus upon roscovitine treatment as shown by subcellular fractionation (F). As expected, UV radiation increased phosphorylation of Chk1 at Ser317 (p-Chk1) (E), and roscovitine decreased Chk1 protein expression and abrogated UV-induced p-Chk1 in both cytoplasm and nucleus (E-F). Proteins 14.3.3 and Sam68 serve as controls for cytoplasmic versus nuclear fraction, respectively. See also Figure S6. (G and H) Transient knockdown of Cep164 inhibits proliferation, which is rescued by wild-type but not mutant CEP164. In clonally selected and doxycycline (Dox)-inducible mouse IMCD3 cells siRNA knockdown was performed. (G) IMCD3 cells depleted of murine Cep164 grew more slowly (siRNA, green line) than nondepleted cells (control, blue line) or the nondepleted cells induced to express human wild-type CEP164 (Dox, red line). Expression of WT Cep164 in siRNA-depleted cells rescued the slow growth phenotype of Cep164 depletion (siRNA+dox, purple line). (H) As in (G), except mutant Cep164 cDNA (CEP164-Q525X) was expressed under doxycyclin control. Expression of this allele itself had a negative impact on cell growth (green line), suggesting a dominant negative effect. An even greater negative effect was seen when the endogenous Cep164 was depleted in cells expressing CEP164-Q525X (siRNA+dox, purple line). The average counts are plotted with standard deviations. Asterisks indicate significant differences by unpaired Student’s t test (p < 0.05). |
Reprinted from Cell, 150(3), Chaki, M., Airik, R., Ghosh, A.K., Giles, R.H., Chen, R., Slaats, G.G., Wang, H., Hurd, T.W., Zhou, W., Cluckey, A., Gee, H.Y., Ramaswami, G., Hong, C.J., Hamilton, B.A., Cervenka, I., Ganji, R.S., Bryja, V., Arts, H.H., van Reeuwijk, J., Oud, M.M., Letteboer, S.J., Roepman, R., Husson, H., Ibraghimov-Beskrovnaya, O., Yasunaga, T., Walz, G., Eley, L., Sayer, J.A., Schermer, B., Liebau, M.C., Benzing, T., Le Corre, S., Drummond, I., Janssen, S., Allen, S.J., Natarajan, S., O'Toole, J.F., Attanasio, M., Saunier, S., Antignac, C., Koenekoop, R.K., Ren, H., Lopez, I., Nayir, A., Stoetzel, C., Dollfus, H., Massoudi, R., Gleeson, J.G., Andreoli, S.P., Doherty, D.G., Lindstrad, A., Golzio, C., Katsanis, N., Pape, L., Abboud, E.B., Al-Rajhi, A.A., Lewis, R.A., Omran, H., Lee, E.Y., Wang, S., Sekiguchi, J.M., Saunders, R., Johnson, C.A., Garner, E., Vanselow, K., Andersen, J.S., Shlomai, J., Nurnberg, G., Nurnberg, P., Levy, S., Smogorzewska, A., Otto, E.A., and Hildebrandt, F., Exome Capture Reveals ZNF423 and CEP164 Mutations, Linking Renal Ciliopathies to DNA Damage Response Signaling, 533-548, Copyright (2012) with permission from Elsevier. Full text @ Cell