- Title
-
Prostaglandin Analogs and Eupatilin as Treatments for Nephronophthisis
- Authors
- Tata, A., Rocha, G., Hureaux, M., Serafin, A.S., Porée, E., Menguy, L., Goudin, N., Cagnard, N., Gréau, L., Fila, M., Briseño-Roa, L., Annereau, J.P., Saunier, S., Benmerah, A.
- Source
- Full text @ Kidney Int Rep
|
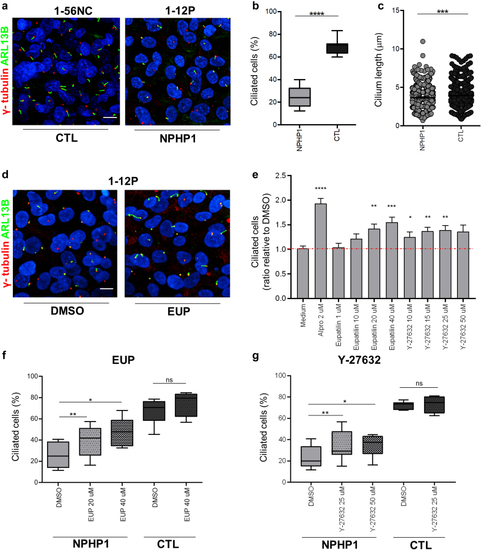
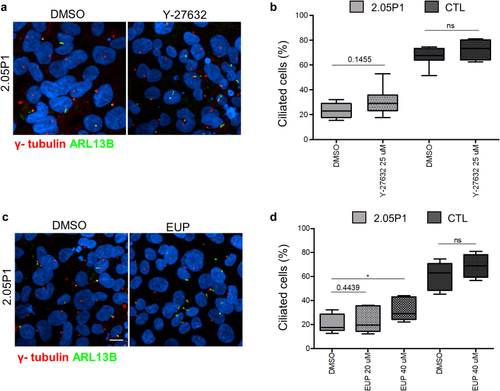
Eupatilin and ROCK inhibitors rescue ciliogenesis defects in |
|
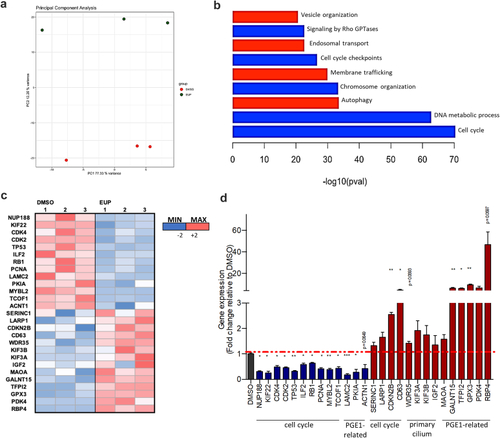
Transcriptomic analyses reveal the cellular pathways modulated in response to Eupatilin treatment. (a) Principal component analysis showing separation between the EUP-treated URECs and the DMSO-treated by PC2 variance. (b) Pertinent down (blue) or up (red) regulated pathways or relevant processes involving ciliary modulators dysregulated in |
|
|
|
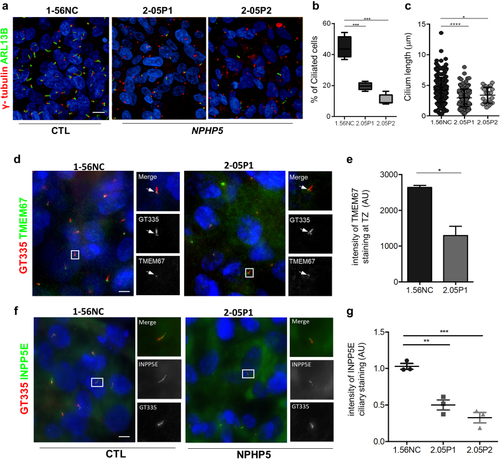
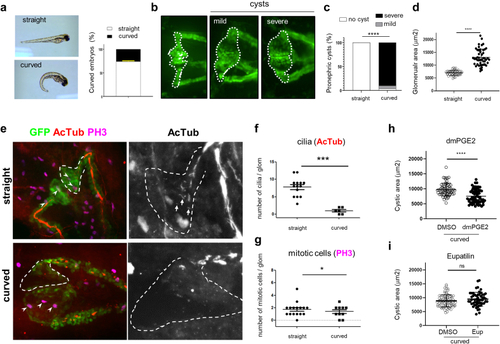
Ciliogenesis defects in |
|
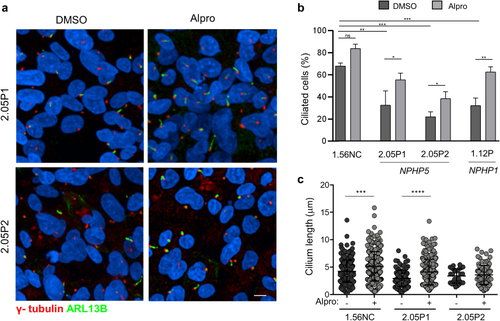
Eupatilin but not Rock inhibitor increases ciliation in |
|
PGE2, but not Eupatilin, shows beneficial effect on cysts size in PHENOTYPE:
|