- Title
-
Mcm5 mutation leads to silencing of Stat1-bcl2 which accelerating apoptosis of immature T lymphocytes with DNA damage
- Authors
- Liu, M., Li, Y., Deng, Z., Zhang, K., Huang, S., Xia, J., Feng, Y., Liang, Y., Sun, C., Liu, X., Li, S., Su, B., Dong, Y., Huang, S.
- Source
- Full text @ Cell Death Dis.
|
|
|
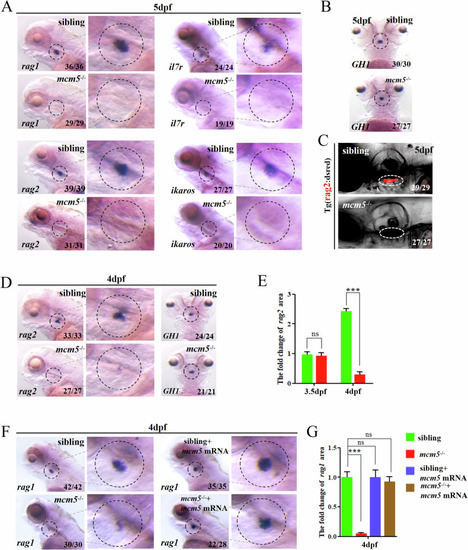
Immature T lymphocytes undergo cell death in |
|
p53-dependentproapoptotic signaling mediates the death of immature T lymphocytes. |
|
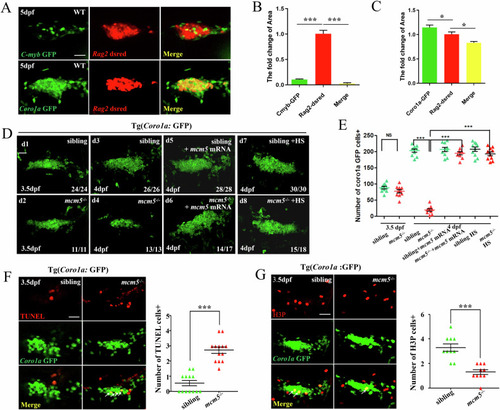
Immature T cells in |
|
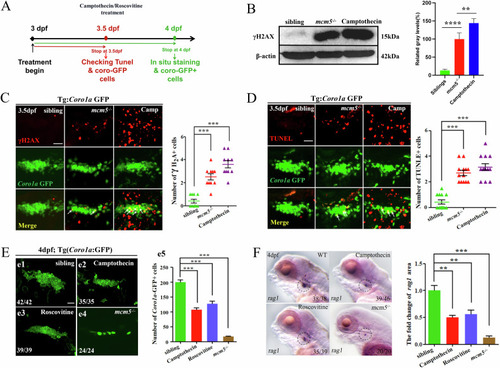
Silencing of |
|
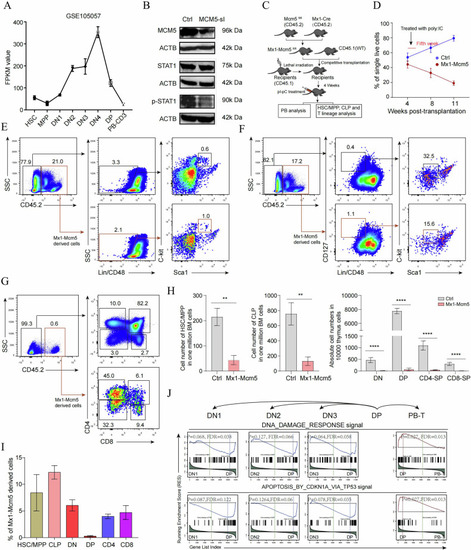
The MCM5/stat1 complex is required for Stat1 phosphorylation and downstream gene |
|
CD4+CD8+ DP cells are most sensitive to Mcm5 knockout. |