- Title
-
The forkhead transcription factor Foxj1 controls vertebrate olfactory cilia biogenesis and sensory neuron differentiation
- Authors
- Rayamajhi, D., Ege, M., Ukhanov, K., Ringers, C., Zhang, Y., Jung, I., D'Gama, P.P., Li, S.S., Cosacak, M.I., Kizil, C., Park, H.C., Yaksi, E., Martens, J.R., Brody, S.L., Jurisch-Yaksi, N., Roy, S.
- Source
- Full text @ PLoS Biol.
|
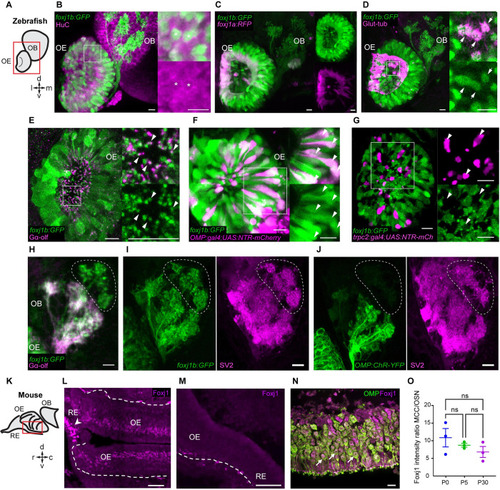
( EXPRESSION / LABELING:
|
|
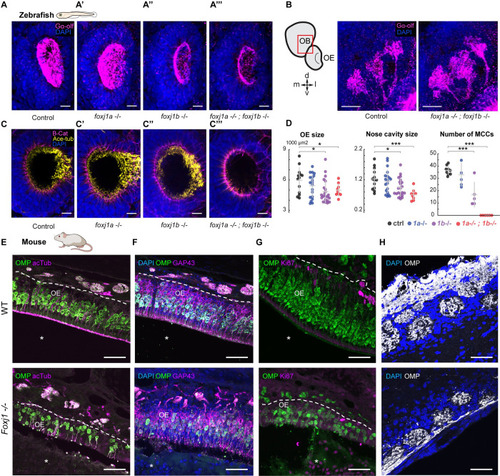
Foxj1 regulates olfactory cilia biogenesis in zebrafish and OE establishment in mice. ( EXPRESSION / LABELING:
PHENOTYPE:
|
|
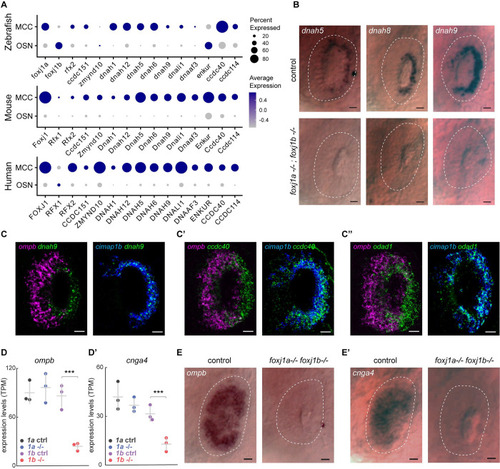
Foxj1 controls the expression of OSN-specific genes, but not ciliary motility genes. ( EXPRESSION / LABELING:
PHENOTYPE:
|
|
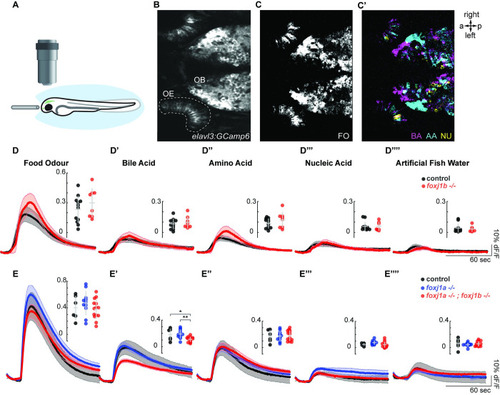
Olfactory response to bile acid is reduced in ( |