- Title
-
CRISPR/Cas9-mediated nexilin deficiency interferes with cardiac contractile function in zebrafish in vivo
- Authors
- Hofeichner, J., Gahr, B.M., Huber, M., Boos, A., Rottbauer, W., Just, S.
- Source
- Full text @ Sci. Rep.
|
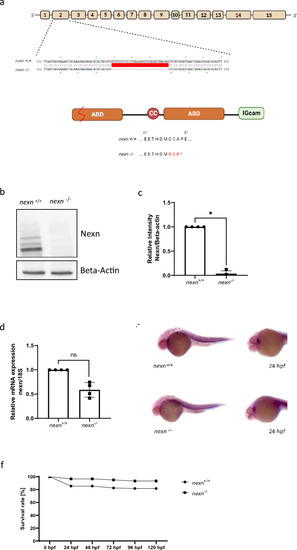
Generation of zebrafish |
|
|
|
|
|
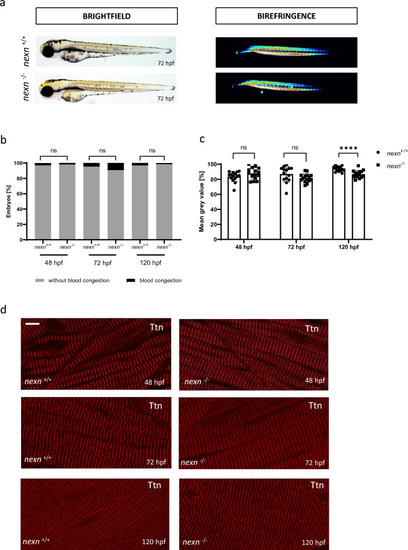
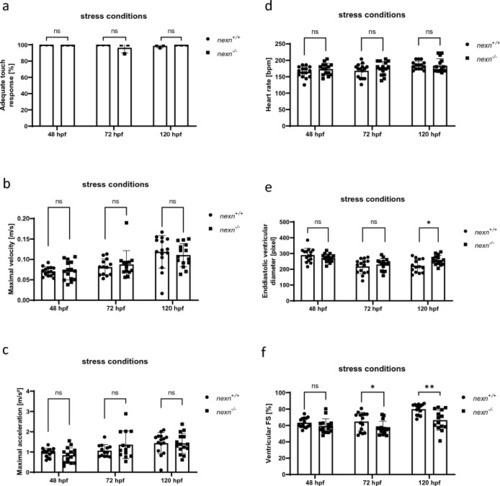
Increased muscular workload causes skeletal muscle disruption in later developmental stages of |
|
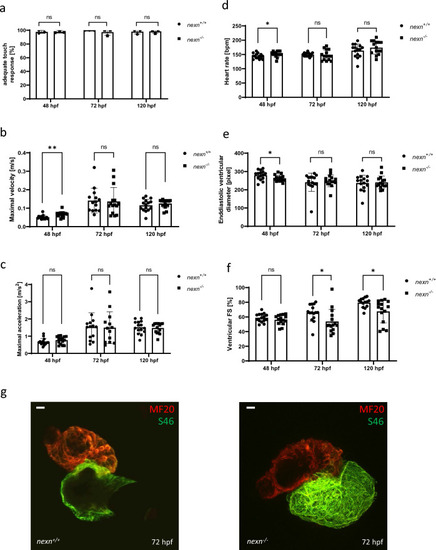
Increased muscular workload does not lead to aggravated cardiac dysfunction in |
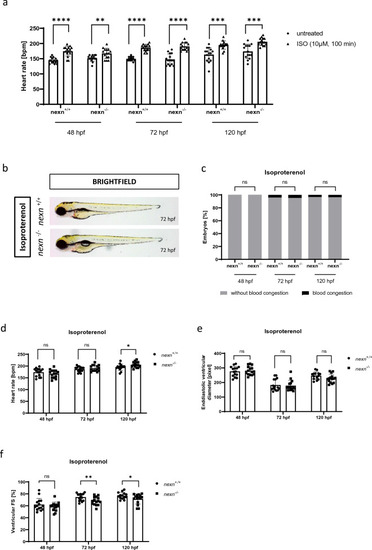
|
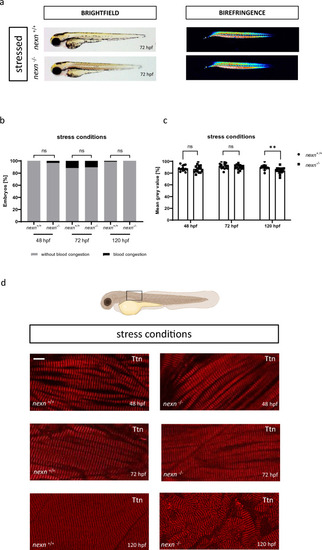
Increasing heart rate does not cause severe changes in cardiac functionality. ( |
|
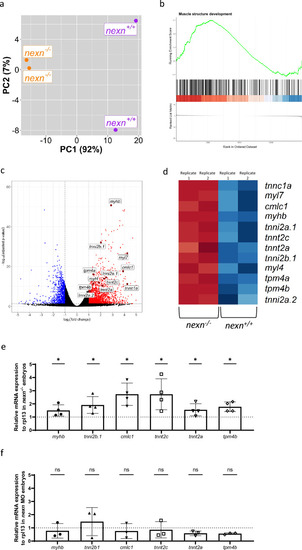
Nexn deficiency leads to upregulation of several genes encoding for sarcomeric proteins. ( |