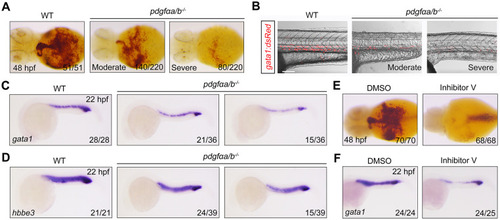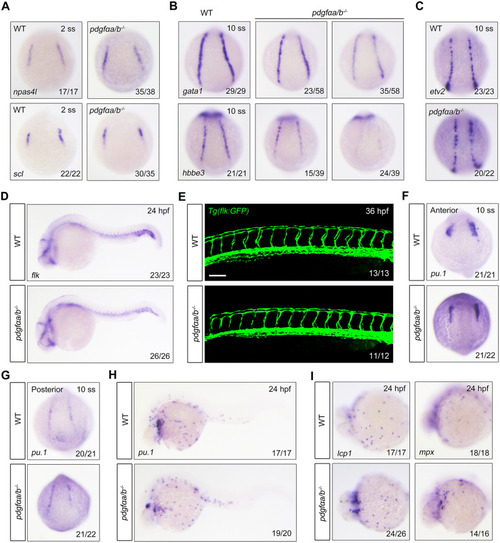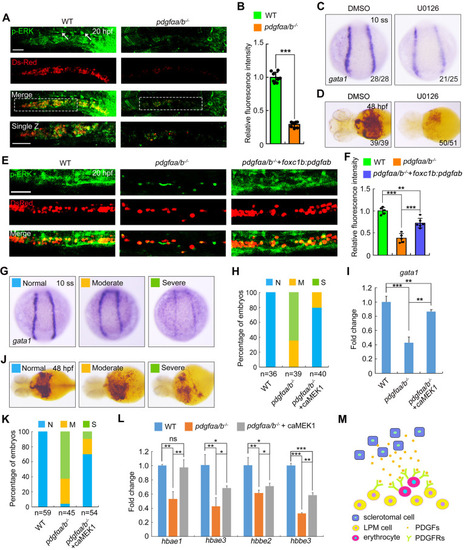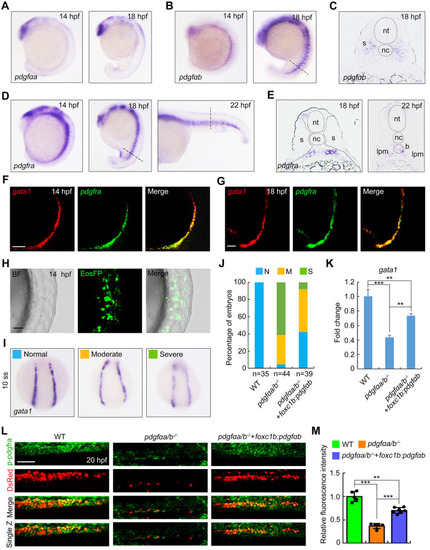
Overexpression of pdgfαb in sclerotome is sufficient to rescue primitive erythropoietic defects in pdgfαa−/−;pdgfαb−/− embryos. (A) Expression analysis of pdgfαa in wild-type embryos at the indicated stages by in situ hybridization. Lateral view with anterior to the top. (B,C) Expression profile of pdgfαb in wild-type embryos assayed by in situ hybridization at the indicated stages and displayed (B) as whole mounts (lateral view with anterior to the top) or (C) in section. Dashed line in B indicates the position of the section in C. (D,E) Expression patterns of pdgfra in wild-type embryos assayed by in situ hybridization at the indicated stages, shown as (D) whole mounts or (E) sections. Dashed lines in D represent the positions of the sections in E. B, blood cell; lpm, lateral plate mesoderm; nc, notochord; nt, neural tube; s, somite. (F,G) Colocalization analysis of pdgfra and gata1 transcripts in wild-type embryos at (F) 14 hpf and (G) 18 hpf by dual-color fluorescence in situ hybridization. Lateral view with anterior to the top. Scale bars: 50 μm. (H-K) pdgfαa−/−;pdgfαb−/− (pdgfαa/b−/−) embryos were injected with 30 pg foxc1b:pdgfαb-P2A-EosFP plasmid and 200 pg Tol2 transposase mRNA at the one-cell stage, and then harvested for in vivo confocal imaging or in situ hybridization alongside uninjected WT and pdgfαa/b−/− control embryos. (H) Representative brightfield (BF) and confocal images of a pdgfαa/b−/− embryo injected with the plasmid foxc1b: pdgfαb-P2A-EosFP and Tol2 mRNA at 14 hpf. Lateral view with anterior to the top. Scale bar: 50 μm. (I) The transcripts of gata1 were evaluated by in situ hybridization at 10-somite stage (10 ss). Representative images of the phenotypes observed in uninjected WT or pdgfαa/b−/− control embryos and pdgfαa/b−/− embryos injected with the indicated plasmid and Tol2 transposase mRNA. (J) The percentage of embryos scored as being normal (N) or as having moderate (M) or severe (S) defects for the indicated genotypes. (K) Quantitative real-time PCR was performed to examine the expression of gata1 in the indicated embryos, and the values are expressed as mean±s.d. Fold change relative to WT from three independent experiments. One-way ANOVA with Scheffe post-hoc test was used to analyze the statistical differences between groups. **P<0.01, ***P<0.001. (L,M) Immunofluorescence assay of p-Pdgfra in Tg(gata1:dsRed) background embryos. WT and pdgfαa/b−/− control embryos were uninjected, and pdgfαa/b−/− embryos were injected with foxc1b:pdgfαb-P2A-EosFP plasmids and Tol2 transposase mRNA as described in H. (L) Lateral views with anterior to the left. Scale bar: 50 μm. (M) The average fluorescence intensity of p-Pdgfra in the ICM region of each group was calculated using ImageJ software, and the fluorescence intensity values relative to WT are shown as mean±s.d. (n>5) from three independent experiments. One-way ANOVA test with Scheffe post-hoc test was used to analyze the statistical differences between groups. **P<0.01, ***P<0.001.
|