- Title
-
Evaluation of Neural Regulation and Microglial Responses to Brain Injury in Larval Zebrafish Exposed to Perfluorooctane Sulfonate
- Authors
- Paquette, S.E., Martin, N.R., Rodd, A., Manz, K.E., Allen, E., Camarillo, M., Weller, H.I., Pennell, K., Plavicki, J.S.
- Source
- Full text @ Environ. Health Perspect.
|
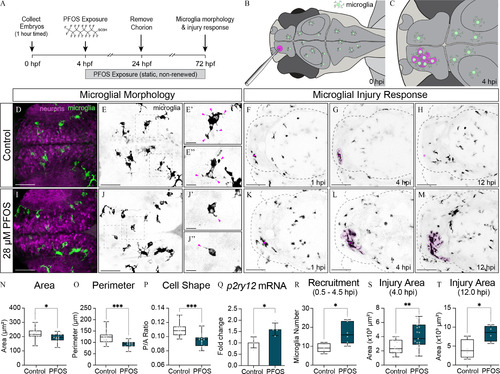
Microglia morphology and response to minor brain injury in 3-dpf larvae exposed to |
|
Optogenetic modulation of PFOS-exposed microglia. (A) Schematic of halorhodopsin: Optogenetic modulation of microglia electrical state is achieved via photostimulation of the light-gated chloride pump, halorhodopsin (eNpHR3.0). eNpHR3.0 is most responsive to |
|
Analysis of regional and global neuronal network activity following chronic exposure to PFOS. (A) Illustrative representation of a larval zebrafish brain with anatomical regions outlined: forebrain (FB), optic tectum (OT), cerebellum (Ce), hindbrain (HB), and whole brain (WB). (B) Illustrative representation of neuron-driven CaMPARI: neurons with low intracellular calcium (low |
|
Assessing anxiety-like swim behavior in PFOS-exposed larvae. (A) Larval swim behavior during a 30-min light/dark behavioral assay was used to determine time spent in the well’s center (crossing center: less anxious) vs. time spent along the well’s edge (center avoidance: more anxious). (B) Heat maps were generated to indicate mobile (blue) vs. stationary (red) swim activity within the well, as well as the zones traversed within the well. (C) Quantification of the time that 3-dpf |
|
Light/dark behavioral assay and neuronal activity in microglia mutants exposed to PFOS. Wild-type (WT) and microglia-deficient |
|
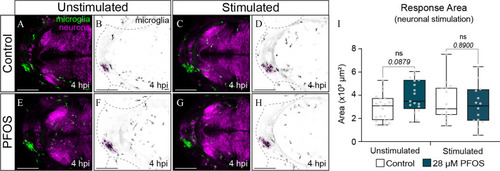
Microglia response to brain injury following optogenetic silencing of neurons in PFOS-exposed larvae. (A) Confocal micrograph of a 3-dpf control-treated larval brain with fluorescently labeled microglia in green and |
|
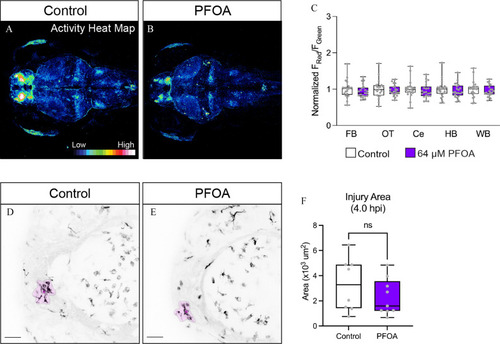
Neuronal activity and microglia response to brain injury following exposure to PFOA, a known immunotoxic congener. PFOA is an 8-carbon PFAS compound with a carboxylate head group. To observe the effects of PFOA on neuronal activity, we again used larvae of the |