- Title
-
The USP46 complex deubiquitylates LRP6 to promote Wnt/β-catenin signaling
- Authors
- Ng, V.H., Spencer, Z., Neitzel, L.R., Nayak, A., Loberg, M.A., Shen, C., Kassel, S.N., Kroh, H.K., An, Z., Anthony, C.C., Bryant, J.M., Lawson, A., Goldsmith, L., Benchabane, H., Hansen, A.G., Li, J., D'Souza, S., Lebensohn, A.M., Rohatgi, R., Weiss, W.A., Weiss, V.L., Williams, C., Hong, C.C., Robbins, D.J., Ahmed, Y., Lee, E.
- Source
- Full text @ Nat. Commun.
|
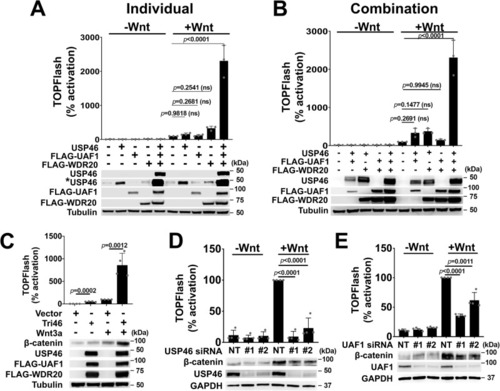
The USP46 complex is a positive regulator of Wnt signaling. |
|
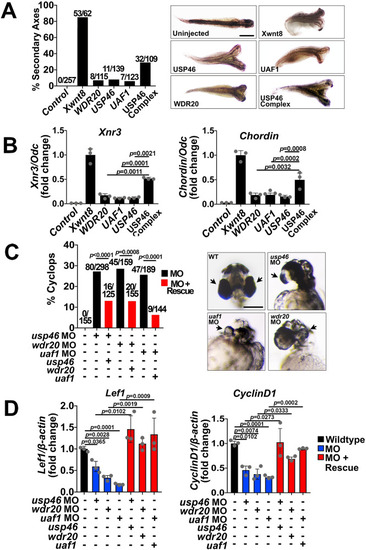
The USP46 complex regulates Wnt signaling in |
|
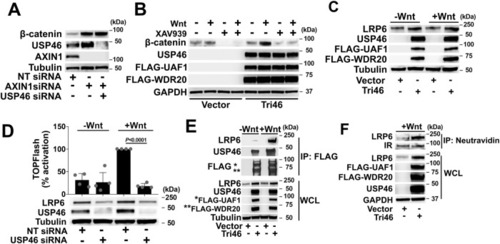
The USP46 complex acts at the level of the Wnt receptor, LRP6. |
|
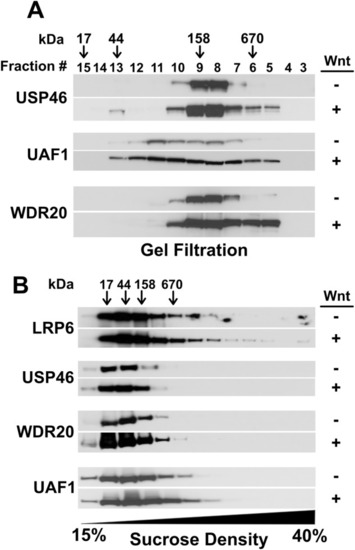
The USP46 complex associates with large complexes independent of LRP6 signalosomes in the presence of Wnt. |
|
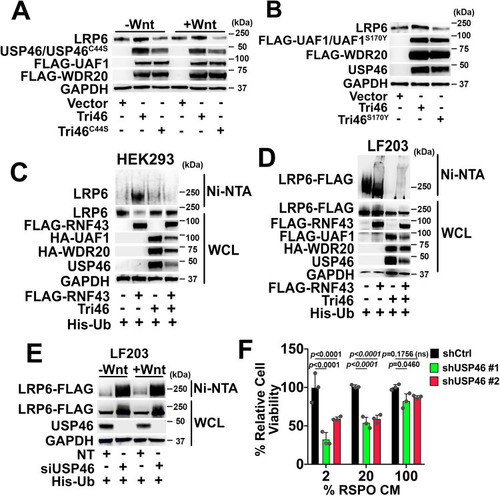
The USP46 complex deubiquitinates LRP6 and opposes the action of the Wnt receptor E3 ligase, RNF43. |
|
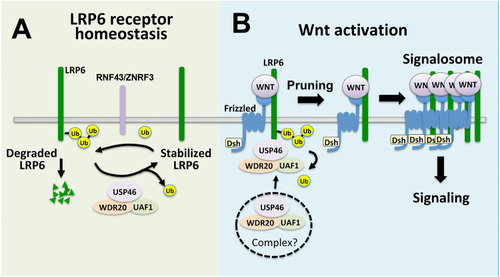
Model of USP46 complex regulation of LRP6 in the Wnt pathway. |