- Title
-
A multi-depth spiral milli fluidic device for whole mount zebrafish antibody staining
- Authors
- Ye, S., Chin, W.C., Ni, C.W.
- Source
- Full text @ Biomed. Microdevices.
|
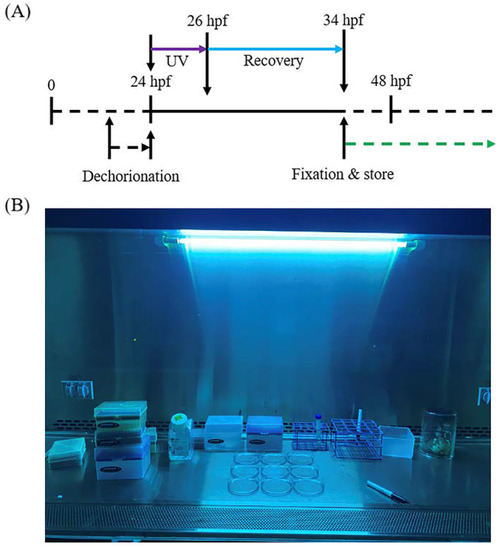
Zebrafish embryo Caspase-3 cleavage activation and sample preparation. |
|
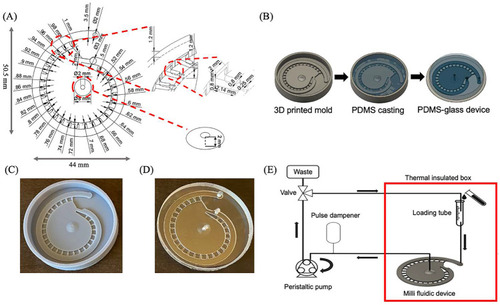
Multi-depth spiral milli fluidic device for zebrafish immobilization and antibody staining. |
|
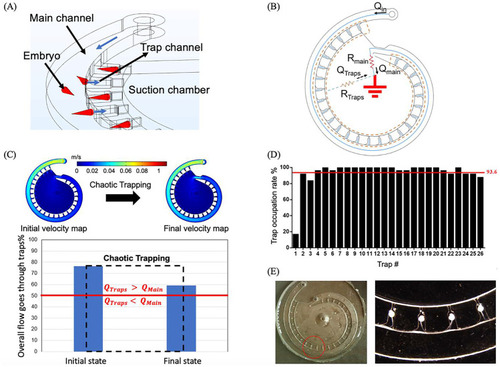
The zebrafish embryo trapping principles and validations. |
|
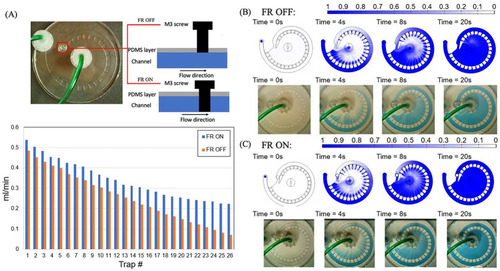
On-chip flow settings for the multi-depth spiral device. |
|
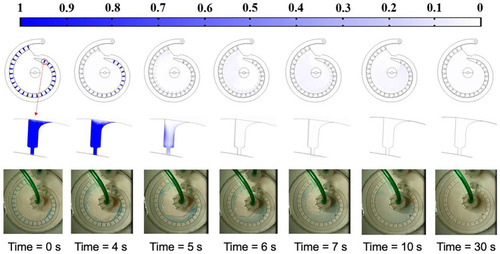
The flushing time estimation. |
|
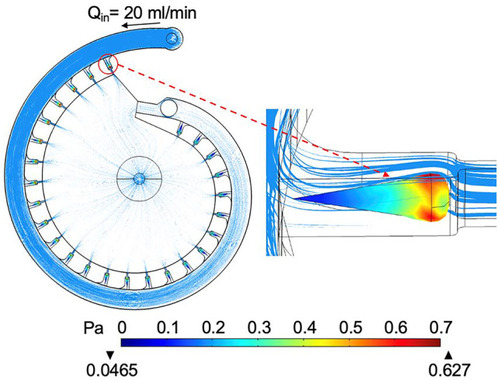
The simulated body shear stress on the fixed zebrafish embryos during on-chip perfusion. The embryo in the first trap is selected to show the maximum shear stress level and the shear stress distribution. The flowrate is set at 20 ml/min and the FR is turned on |
|
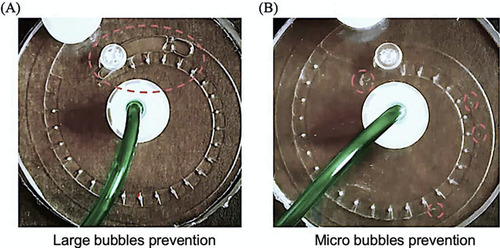
Bubble prevention in the milli fluidic device. |