- Title
-
Cfdp1 Is Essential for Cardiac Development and Function
- Authors
- Giardoglou, P., Deloukas, P., Dedoussis, G., Beis, D.
- Source
- Full text @ Cells
|
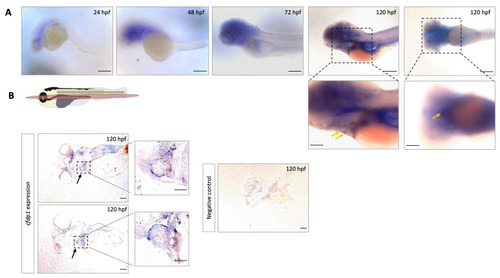
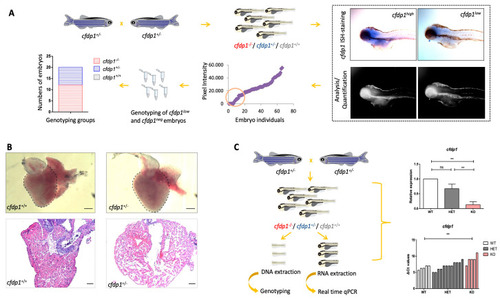
Expression analysis of |
|
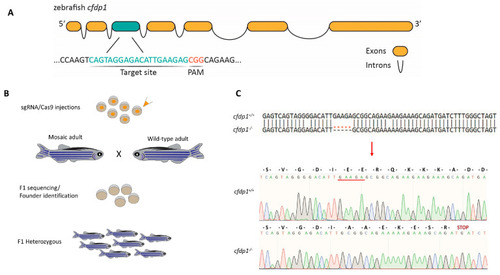
Generation of |
|
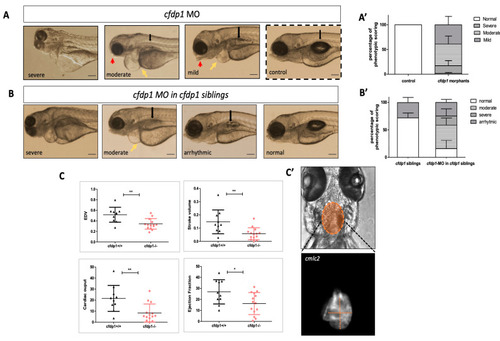
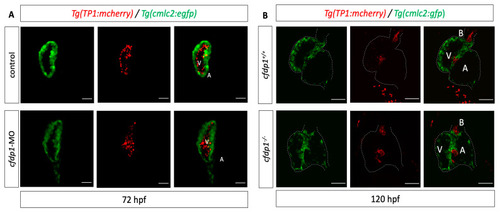
Impaired cardiac performance of |
|
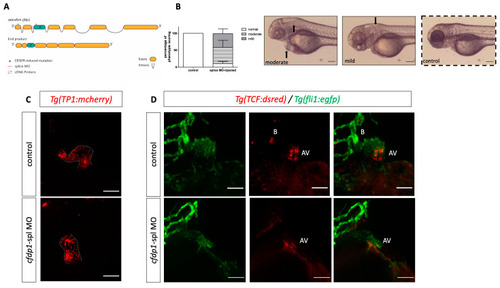
Silencing of EXPRESSION / LABELING:
PHENOTYPE:
|
|
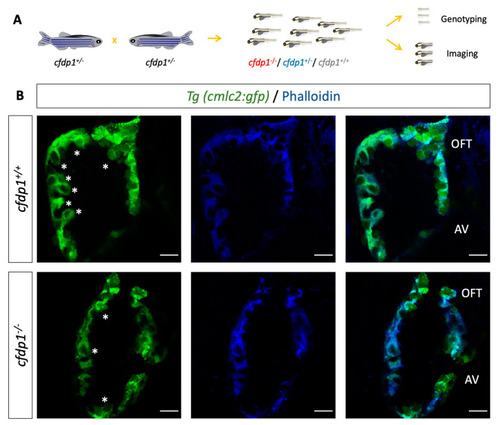
Study of EXPRESSION / LABELING:
PHENOTYPE:
|
|
PHENOTYPE:
|
|
PHENOTYPE:
|
|
PHENOTYPE:
|

Unillustrated author statements |