- Title
-
Monitoring Nrf2/ARE Pathway Activity with a New Zebrafish Reporter System
- Authors
- Badenetti, L., Manzoli, R., Rubin, M., Cozza, G., Moro, E.
- Source
- Full text @ Int. J. Mol. Sci.
|
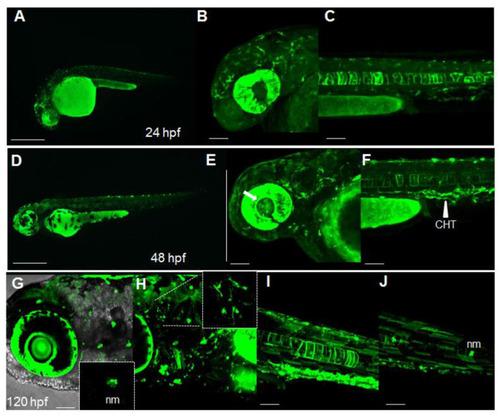
Dynamic spatiotemporal reporter expression in the Nrf2/ARE transgenic line. (A) Whole-mount fluorescence microscopy of a Tg(8xAORE:EGFP)ia201 larva 24 hpf, showing the widespread expression of reporter-expressing cells in both cephalic and more caudal regions. (B,C) Confocal Z-stack projection of the cephalic area (B) and trunk region (C) of a 24 hpf transgenic larva in which strong fluorescent cells are detectable in the brain, eye and notochord. (D) Whole-mount fluorescence microscopy of a 48 hpf Tg(8xAORE:EGFP)ia201 larva, showing the prolonged expression of the reporter in the cephalic and trunk regions. (E,F) Confocal Z-stack projection of the cephalic area (E) and trunk regions (F) of the same 48 hpf transgenic larva exhibiting novel expression domains in the lens and inner eye (white arrow) and the caudal hematopoietic tissue (CHT) (white arrowhead). (G?J) Confocal Z-stack projection of the cephalic area (G,H) and trunk regions (I,J) of a 120 hpf transgenic larva showing fluorescent neuromasts (nm) and protrusion-carrying brain cells (small inset in H), as well as muscle fibers along the trunk (I,J). All images are lateral views with anterior to the left. Scale bar in (A,D): 500 ?m; in (B,C,E,F) and (G?J): 100 ?m. |
|
Pharmacological validation of Nrf2/ARE reporter fish. (A) Whole-mount bright field and fluorescence microscopy acquisition of a 24 hpf transgenic larva treated with DMSO and the Nrf2 pathway agonist, RTA-408, for 16 h. A significant increase in fluorescent cells is detected in RTA-408-treated larvae when compared to DMSO-treated fish. (B) Whole-mount bright field and fluorescence microscopy acquisition of a 24 hpf transgenic larva treated with DMSO and the Nrf2 pathway antagonist, Brusatol, for 16 h. A visible decrease in reporter fluorescent intensity is visible in Brusatol-treated larvae. All images are lateral views with anterior to the left. The magnifications of the trunk regions are confocal Z-stack acquisitions. The graphs reported on the right depict the ImageJ-based quantification of the selected trunk region of 5 independently treated fish (* p < 0.05; ** p < 0.005, t-test). Scale bars in the panels with multiple fish: 500 ?m; scale bars on the panels with a single magnified larva: 100 ?m. |
|
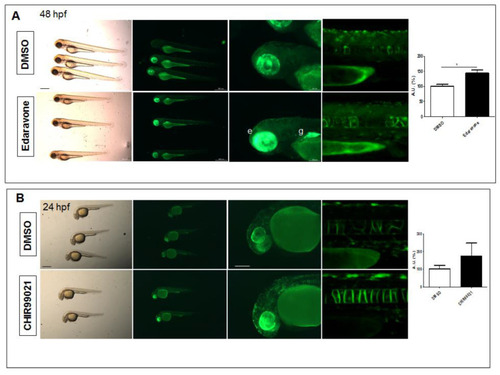
Pharmacological testing of Nrf2/ARE reporter fish. (A) Whole-mount bright field and fluorescence microscopy acquisition of 48 hpf transgenic larvae treated with DMSO or the radical scavenger Edaravone for 24 h. An evident increase in fluorescence is detected in the eye (e) and gut (g) of Edaravone-treated larvae when compared to DMSO-treated control fish. (B) Whole-mount bright field and fluorescence microscopy acquisition of 24 hpf transgenic larvae treated with DMSO or the GSK3? antagonist, CHIR99021, for 16 h. Increased reporter fluorescence is observed throughout the whole larvae when compared to the DMSO-treated control fish. The magnifications of the trunk regions are confocal Z-stack acquisitions. The graphs reported on the right depict the ImageJ-based quantification of the selected trunk region of 5 independent treated fish. (* p < 0.05, t-test) All images are lateral views with anterior to the left. Scale bars in the panels with multiple fish: 500 ?m; scale bars on the panels with a single magnified larva: 100 ?m. |
|
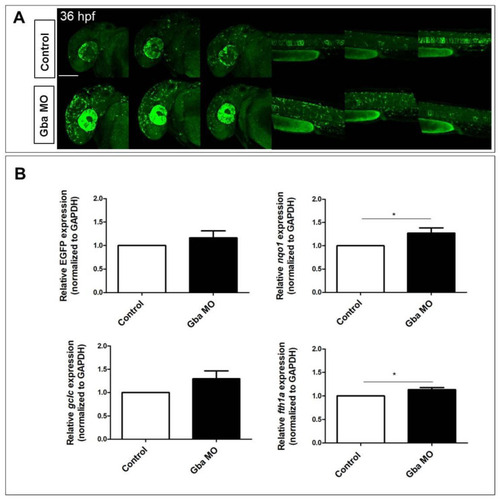
Beta glucocerebrosidase knockdown does not significantly increase Nrf2/ARE reporter expression but does induce the upregulation of Nrf2-pathway-related targets. (A) Confocal Z-stack projections of 30 hpf control and Gba1 morphant fish, showing increased reporter expression in both cephalic and caudal regions of morphants. For comparison, three different morphant and control fish are depicted. All images are lateral view with anterior to the left. Scale bar: 100 ?m. (B) Bar graphs showing the quantitative EGFP reporter and Nrf2 pathway-related genes expression measured in morphant and control fish RNA extracts. Data are mean ± SD of three independent biological replicates (each replicate consists of ten larvae per condition; * p < 0.05; t-test). |
|
Nrf2/ARE pathway activity increases in regenerating tail fins. Representative Z-stack projection of a confocal fluorescence microscopy acquisition of unamputated and amputated tail fin of representative 4 dpf and 7 dpf Nrf2/ARE transgenic fish after at 24 h post amputation (hpa), showing fluorescent cells migrating along the stump (white arrowheads). Scale bar: 100 ?m. |