- Title
-
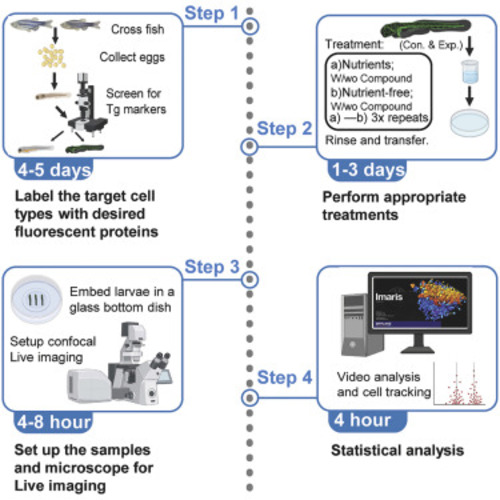
Optimized protocol for live imaging of overnutrition-elicited interactions between immune cells and β cells in zebrafish
- Authors
- Yang, B., Zhang, Y., Yang, L., Chen, W.
- Source
- Full text @ STAR Protoc
|
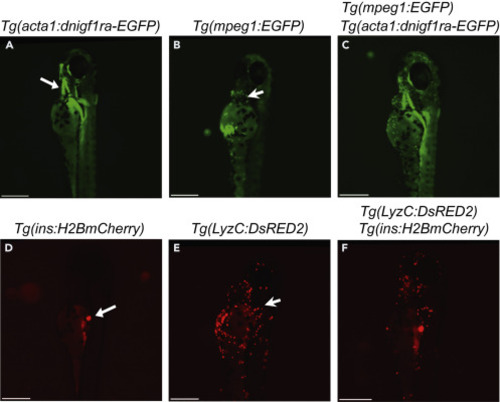
Identify fish with the desired genotypes (A) Image of Tg(acta1:dnigf1ra-EGFP)(zMIR ) fish at 4 dpf that expresses a dominant negative Igf1r-EGFP indicated by green immunofluorescence in skeletal muscle, particularly the jaw muscle (White arrow). (B) Image of Tg(mpeg1:EGFP) fish at 4 dpf with its macrophages labelled by EGFP (Green dots). The white arrow points to a macrophage. (C) Image of a zMIR larva that also has EGFP-labelled macrophages. (D) Image of a Tg(ins:H2B-mCherry) fish at 4 dpf showing red fluorescence in the β cells (White arrow). (E) Image of a Tg(lyzC:DsRed2) fish at 4 dpf showing red fluorescence in neutrophils (Red dot). The white arrow points to a neutrophil. (F) Image of a larva that has Tg(ins:H2B-mCherry) and Tg(lyzC:DsRed2). All scale bars represent 100 μm. |
|
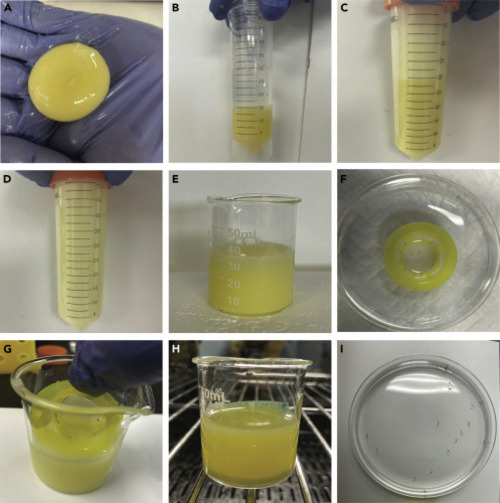
Prepare and use the nutrient-rich medium (A) Harvest egg yolk. (B) Add equal amount of 0.3x Danieau’s solution to an egg yolk-containing conical tube. (C) Generate 50% stock by vigorous vortexing. (D) Prepare working medium by vigorous mixing of 5 mL Stock and 45 mL 0.3x Danieau’s solution. (E) Add 25 mL of the 5% egg yolk medium and < 20 larvae to a clean 50 mL beaker. (F) Transfer the larvae into the Sterile Cell Strainer in a Petri dish. (G) Quickly transfer the larvae into a 50 mL clean beaker with 25 mL of the 5% egg yolk medium. (H) Put the beaker into a 28°C incubator. As sediments accumulate, stir the medium every 2 h (I) Transfer the larvae clear of egg yolk into a Petri dish with 0.3x Danieau’s solution. |
|
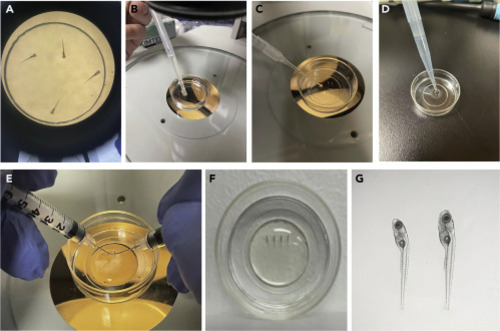
Embed larvae in LMA for imaging (A) Transfer the larvae into a 35 mm Glass Bottom Dish. (B) After anesthesia, remove most of the medium with a transfer pipette. (C) Remove the residual medium using a transfer pipette with a 10 μL pipette tip attached. (D) Add 750 μL of the pre-heated 1.2% LMA at the center of glass bottom using a P1000 pipette. (E) Position the larvae on their right side using two insulin syringe needles. (F) Add 750 μL 0.3x Danieau’s solution (containing Tricaine) at the periphery of the dish after the LMA solidifies to submerge the gel. (G) Check the larvae under a stereo microscope. |
|
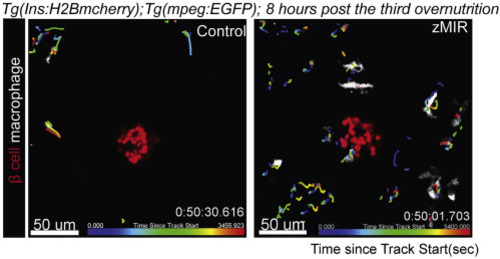
Track macrophages near the principal islet in control and zMIR fish from 64 to 65 h post treatment using Imaris The islet is labelled by H2B-mCherry (red) and macrophages are labelled by EGFP (white). Each track is color coded to indicate the time during which the macrophage was tracked. The color gradient at the bottom of each image represent the tracking time. |
|
|