- Title
-
Splicing factor deficits render hematopoietic stem and progenitor cells sensitive to STAT3 inhibition
- Authors
- Potts, K.S., Cameron, R.C., Metidji, A., Ghazale, N., Wallace, L., Leal-Cervantes, A.I., Palumbo, R., Barajas, J.M., Gupta, V., Aluri, S., Pradhan, K., Myers, J.A., McKinstry, M., Bai, X., Choudhary, G.S., Shastri, A., Verma, A., Obeng, E.A., Bowman, T.V.
- Source
- Full text @ Cell Rep.
|
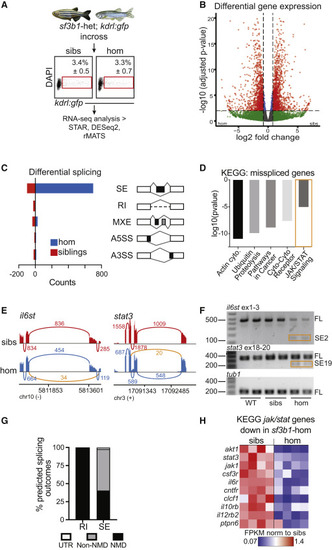
Stat3 pathway components are differentially spliced and expressed in sf3b1 mutant zebrafish cells (A) Experimental setup for zebrafish RNA sequencing. kdrl:gfp+ endothelial cells were FACS-isolated from 24 h post fertilization (hpf) embryos (sf3b1 homozygous mutants [hom] and siblings [sibs]). (B) Volcano plot of differentially expressed genes from RNA-seq analysis comparing sf3b1 homozygous mutants and siblings. Significance thresholds imposed at adjusted p < 0.01 and log2 fold change > |0.8|. (C) Quantification of differential splicing events between sf3b1 homozygous mutants and siblings, separated by type of splicing event. SE, cassette (skipped) exon; RI, retained intron; MXE, mutually exclusive exon; A5SS, alternative 5′ splice site; A3SS, alternative 3′ splice site. Events were considered significant if FDR < 0.05 and inclusion difference > |0.1|. (D) KEGG pathway analysis of alternatively spliced genes identified in RNA-seq analysis of zebrafish sf3b1 homozygous mutants versus siblings. Cyto, cytokine. (E) Sashimi plots showing exon-skipping events identified in il6st (exon 3) and stat3 (exon 19), derived from rMATS analysis of RNA-seq data of endothelial cells from sf3b1 homozygotes (blue) versus siblings (red) at 24 hpf. Alternative splicing event identified in homozygotes indicated (orange). Numbers on plots depict average number of reads across replicates supporting the event. (F) RT-PCR for il6st (top) and stat3 (middle) splice isoforms and tubulin (tub1) control (bottom) from purified endothelial cells from 24 hpf WT, siblings, and homozygous sf3b1 mutant embryos. SE2, il6st ex2 skipped exon isoform; SE19, stat3 ex19 skipped exon isoform; FL, full-length isoform, including upstream, downstream, and skipped exons; tub1, tubulin control. Representative gel images of n = 3 experiments. (G) Graph depicting the percentage of alternatively spliced isoforms (RI, retained introns; SE, skipped exons) in sf3b1 mutants that are predicted to result in nonsense-mediated (NMD) decay, protein sequence alterations (non-NMD), or changes in untranslated regions (UTR). (H) Expression levels of Jak/Stat pathway components and target genes in sf3b1 homozygotes (right) versus siblings (left) from RNA-seq analysis. FPKM, fragments per kilobase per million mapped reads. Each heatmap column represents a separate biological replicate. See also Figure S1. |
|
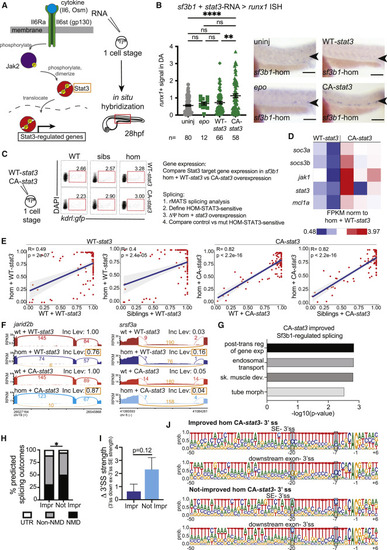
Constitutively active Stat3 signaling partially restores HSPC production in sf3b1 mutants (A) Schematic of Jak/Stat signaling pathway (left). Genes overexpressed are indicated (orange box). Experimental strategy (right): in-vitro-transcribed RNA of target genes was injected into sf3b1 heterozygote incross zebrafish embryos (one-cell stage), then samples fixed at 28 hpf for in situ hybridization using HSPC marker runx1. (B) Representative in situ hybridization (ISH) images of runx1 signal in dorsal aorta (DA) (arrowheads) of sf3b1 homozygous (hom) embryos (right) and quantification of DA runx1 signal in embryos with dots representing individual embryos (left). In-vitro-transcribed RNA injected included: erythropoietin (epo), WT (WT)-stat3, or constitutively active (CA)-stat3. ANOVA; ns, not significant, ∗∗p < 0.01, ∗∗∗∗p < 0.0001. Scale bar, 100 μM. (C) Experimental strategy schematic: in-vitro-transcribed RNA (WT-stat3 or CA-stat3) was injected into one-cell stage embryos derived from sf3b1 heterozygote or WT incrosses, then 24 hpf kdrl:gfp+ endothelial cells were FACS-isolated from each group. RNA-seq data were subjected to gene expression and rMATS splicing analyses. Differential splicing events (FDR < 0.05) were identified between homozygotes injected with WT-stat3 and CA-stat3 (defined as HOM-STAT3 sensitive), then these events were compared in homozygotes versus controls (WT or siblings) injected with WT-stat3 or CA-stat3. (D) Gene expression heatmap of selected Stat3 targets in RNA-seq data from the sf3b1 homozygote rescue experiment. FPKM normalized to homozygotes + WT-stat3. FPKM, fragments per kilobase of transcript per million mapped reads. Each heatmap column represents a separate biological replicate. (E) Scatterplots displaying mean inclusion levels for HOM-STAT3-sensitive skipped exon events in WT or sibling controls and sf3b1 homozygotes injected with WT-stat3 (control, left) or CA-stat3 (right). Solid blue line represents the correlation coefficient between samples with the gray-shaded area denoting the 95% confidence interval. p values calculated for Pearson’s correlation coefficient(R). (F) Sashimi plots showing exon inclusion levels in jarid2b and srsf3a in WT and sf3b1 homozygotes injected with CA-stat3 (lower) compared with WT-stat3 (upper). Expression of CA-stat3 in sf3b1 homozygotes significantly improved exon inclusion in both transcripts toward WT + WT-stat3 levels (orange box). Inc Level is the average inclusion for the indicated splicing event (orange). Numbers on plots depict average number of reads across replicates supporting the event. (G) Pathway analysis of genes with improved splicing in sf3b1 homozygous mutants with CA-stat3 overexpression. post-trans, post-transcriptional; reg, regulation; exp, expression; sk, skeletal; de, development; morph, morphogenesis. (H) Graph depicting the percentage of alternative exon-skipping isoforms in sf3b1 homozygous mutants that were improved or not by CA-stat3 expression that are predicted to result in nonsense-mediated (NMD) decay, protein sequence alterations (non-NMD), or changes in untranslated regions (UTR). Chi-square; ∗p < 0.05. (I) Graph depicting the difference (Δ) in 3′ splice site (ss) strengths between the competing downstream and skipped exons in HOM-STAT3-sensitive SE events that were improved or not by CA-stat3 expression. Student’s t test. (J) Weblogo representations of the 3′ ss and flanking sequences in the competing downstream and skipped exons in the SE events that were improved or not by CA-stat3 expression. Highlighted blue areas show different base preferences in not-improved SE events compared with the other groups. Sequences shown are 50 intronic +6 exonic bases relative to the intron-exon junction. Height of each base represents the relative proportion at the noted position. For all graphs, error bars are standard error mean (SEM). See also Figure S2. |
|
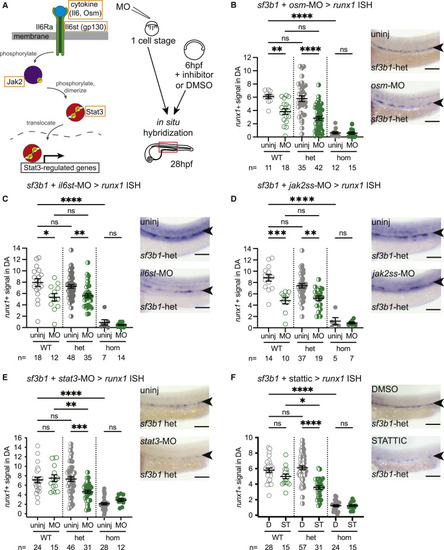
Zebrafish Sf3b1 heterozygous mutant HSPCs are sensitive to STAT3 inhibition (A) Schematic of Jak/Stat signaling pathway (left). Genes targeted for knockdown are indicated (orange box). Experimental strategy (right): target genes were knocked down in zebrafish embryos derived from sf3b1 heterozygote incrosses via either morpholino (MO) injection (one-cell stage) or small-molecule inhibitor administration (6–28 hpf), then samples were fixed at 28 hpf for in situ hybridization with HSPC marker runx1. (B–F) Representative in situ hybridization images of runx1 signal in dorsal aorta (DA) (arrowheads) of sf3b1 heterozygous (het) embryos (right) and quantification of DA runx1 signal in embryos with dots representing individual embryos (left). Targets tested include: ligand osm via morpholino (B); co-receptor il6st via morpholino (C); jak2 via splice-site (ss) morpholino (D); and effector stat3 via splice-site morpholino (E) or with inhibitor stattic (10 μM) versus DMSO control (F). ANOVA; ns, not significant; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. Scale bar, 100 μM. For all graphs, error bars are standard error mean (SEM). See also Figures S3–S5. |
|
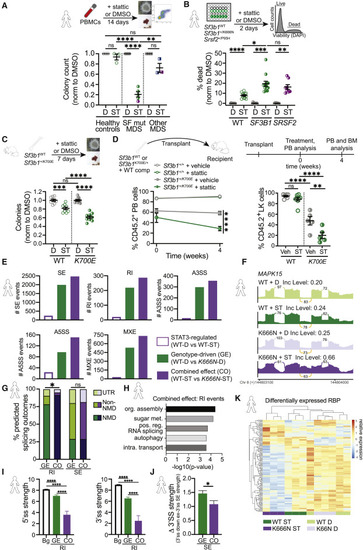
Figure 4. STAT3 inhibition impacts splicing factor mutant mammalian HSPC viability and splicing (A) Experimental strategy schematic (top): human H4434 methylcellulose colony-forming unit (CFU) assay of peripheral blood mononuclear cells (PBMCs) isolated from healthy controls (open circles), splicing factor only mutant MDS patients (filled circles; SF3B1, green; SRSF2, purple), and other mutant MDS patients (filled squares; non-splicing factor mutations only, blue; non-splicing factor + SRSF2 mutations, purple) treated with STAT3 inhibitor stattic (1 μM) or DMSO control. Colonies were counted after 14 days (bottom). Values normalized to DMSO control. n = 3 healthy controls, n = 4 splicing factor mutant MDS, n = 3 other mutant MDS. ANOVA; ns, not significant, ∗∗p < 0.01, ∗∗∗∗p < 0.0001. (B) Experimental strategy schematic (top): isogenic human K562 cell lines with MDS-associated SF3B1+/K666N or SRSF2+/P95H point mutations or WT control were treated with stattic (ST) (2 μM) or DMSO (D) control. Viability (DAPI exclusion) was analyzed by flow cytometry after 2 days of culture. Values normalized to DMSO control (viability). n = 8. ANOVA; ∗p < 0.05, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. (C) Experimental strategy schematic (top): mouse M3434 methylcellulose colony-forming units (CFU) assay of cKit-enriched bone marrow cells isolated from Mx1-Cre:Sf3b1+/+ or Mx1-Cre:Sf3b1+/K700E mice treated with stattic (0.75 μM) or DMSO control. Colonies were counted after 7 days (bottom). Values normalized to DMSO control. n = 8. ANOVA; ns, not significant, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. (D) Experimental strategy schematic (top): 4 weeks after pIpC induction, unfractionated bone marrow cells from either Mx1-Cre:Sf3b1+/+ or Mx1-Cre:Sf3b1+/K700E CD45.2+ mice were competitively transplanted into lethally irradiated CD45.1+ recipients. After reconstitution (time = 0 weeks), treatment with vehicle or stattic was performed for 4 weeks. Quantification of CD45.2+ donor chimerism (bottom) in peripheral blood (left) and BM LK population (right) in recipients treated with vehicle or stattic. n = 5–8 mice per group. ANOVA; ns, not significant, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001. (E) Human K562 cells (SF3B1WT or SF3B1+/K666N) were treated with stattic (10 μM, 24 h) then total RNA was isolated for RNA-seq and analyzed using rMATS. Quantitation of alternative splicing events occurring in each condition comparison (STAT3-regulated, genotype-driven, and combined effect of STAT3 and genotype), separated by type of splicing event. STAT3-regulated genes were significantly altered between SF3B1WT cells treated with stattic versus DMSO. Genotype-driven is defined as genes found differentially spliced between SF3B1WT and SF3B1+/K666N cells treated with DMSO. Combined effect was a comparison of SF3B1WT cells + stattic versus SF3B1+/K666N cells + stattic. SE, cassette (skipped) exon; RI, retained intron; A3SS, alternative 3′ splice site; A5SS, alternative 5′ splice site; MXE, mutually exclusive exon; ST, stattic; D, DMSO. (F) Sashimi plot of combined effect RI event in MAPK15 in SF3B1WT (WT) or SF3B1+/K666N (K666N) K562 cells treated with DMSO (D) or STAT3 inhibitor stattic (ST). Stattic treatment had a more pronounced impact on intron retention (orange) in K666N mutant cells than WT cells. Inc Level is the average inclusion for the impacted intron retention event (orange). Numbers on plots depict average number of reads across replicates supporting the event. (G) Graph depicting the percentage of alternatively spliced isoforms (RI, retained introns; SE, skipped exons) in genotype-driven (GE) and combined effect (CO) splicing events that are predicted to result in nonsense-mediated (NMD) decay, protein sequence alterations (non-NMD), or changes in untranslated regions (UTR). Student’s t test; ns, not significant, ∗p < 0.05. (H) Pathway analysis of combined effect genes significantly altered resulting in retained intron (RI) in SF3B1+/K666N cells in the stattic-treated samples. (I) Graphs showing the average 5′ ss (left) and 3′ ss (right) strengths in control background (Bg) sequences flanking genotype-driven (GE) and combined effect (CO) RI events. ANOVA; ∗∗∗∗p < 0.0001. (J) Graph depicting the difference (Δ) in 3′ ss strength between the competing downstream and skipped exon 3′ ss in genotype-driven (GE) and combined effect (CO) SE events. Student’s t test; ∗p < 0.05. (K) Expression levels of RNA binding proteins differentially expressed between DMSO (D) versus STAT3 inhibitor stattic (ST)-treated SF3B1WT (WT) or SF3B1+/K666N (K666N) K562 cells. Each row represents the relative expression of an individual gene across samples and each column represents a separate biological replicate color coded according to genotype + drug treatment. Genes with an adjusted p < 0.05 between stattic versus DMSO samples are shown. For all graphs, error bars are standard error mean (SEM). See also Figures S6 and S7. |