- Title
-
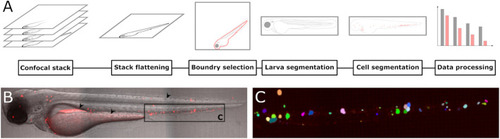
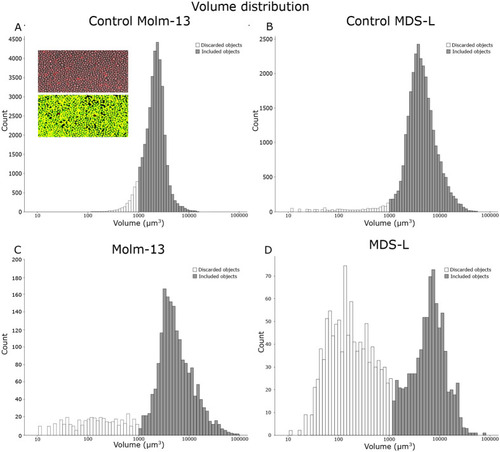
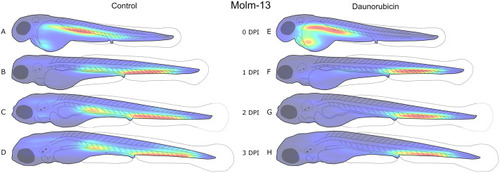
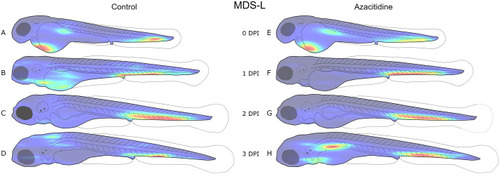
A new software tool for computer assisted in vivo high-content analysis of transplanted fluorescent cells in intact zebrafish larvae
- Authors
- Førde, J.L., Reiten, I.N., Fladmark, K.E., Kittang, A.O., Herfindal, L.
- Source
- Full text @ Biol. Open
|
|
|
|
|
|
|
|
|
|
|
|
|
|