- Title
-
De novo disruptive heterozygous MMP21 variants are potential predisposing genetic risk factors in Chinese Han heterotaxy children
- Authors
- Qin, X.J., Xu, M.M., Ye, J.J., Niu, Y.W., Wu, Y.R., Xu, R., Li, F., Fu, Q.H., Chen, S., Sun, K., Xu, Y.J.
- Source
- Full text @ Hum. Genomics
|
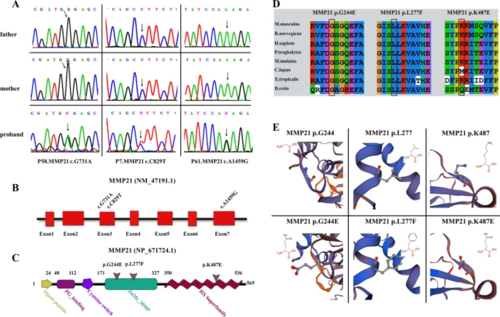
Validation and in silico analysis of MMP21 variants found in HTX patients with complex CHDs. A Sanger sequencing chromatograms of three heterozygous missense MMP21 variants in patients with HTX and complex CHDs and the corresponding normal sequences in related parents. Arrows represent heterozygous nucleotide changes. B, C Schematic diagrams of MMP21 gene and protein showing the location of the identified MMP21 variants. The base substitutions are in exon3 and exon7, respectively (B). The amino acids consist of the domains of the ZnMC_MMP and HX superfamily (C). D Clustal X alignment of amino acid mutations in MMP21 among different species indicates that all mutations are highly conserved in vertebrates. E Schematic diagram of the three-dimensional structure and corresponding amino acid residues at the mutation sites of WT (left panel) and mutant (right panel) MMP21 proteins that were reconstructed by SWISS-MODEL. Amino acid substitutions in the variants p.G244E and p.K487E resulted in changes in acidity or basicity, which may affect the function of the mutant domain. The amino acid residue in the original position of the variant p.L277F was replaced by a residue that occupies a larger space volume, which may affect the stability of the protein spatial structure |
|
The variants do not disrupt the expression of MMP21. The blank (pCMV3), MMP21 WT, or mutant vectors (Mut1, c.G731A; Mut2, c.C829T; Mut3, c.A1459G) were separately transfected or co-transfected (WT/mutant = 1:1) into HEK-293 T cells. A, B Relative mRNA expression of MMP21 in HEK-293 T cells transfected with plasmids for 36 h was assessed by RT-qPCR. 18sRNA was used as an internal control. The RNA levels in the WT group were set at 1. There was no significant difference in MMP21 mRNA expression between the WT and mutant groups (all data are shown as the mean ± SEM, and one-way ANOVA with Tukey’s multiple comparisons test was performed for statistical calculation of MMP21 mRNA expression between the WT and mutant groups, n = 3 independent experiments). C, D Relative MMP21 protein expression in HEK-293 T cells transfected with plasmids for 48 h was evaluated using western blot analysis. β-Actin was used as an internal control (left panel). The intensity quantitation of MMP21 WT and mutant protein expression levels were normalized to β-actin expression using ImageJ software (right panel). Quantitative results revealed no significant difference in MMP21 protein expression between WT and mutant groups (all data are shown as the mean ± SEM; one-way ANOVA with Tukey’s multiple comparisons test was used for statistical calculation of MMP21 protein expression between WT and mutant groups, n = 3 independent experiments) |
|
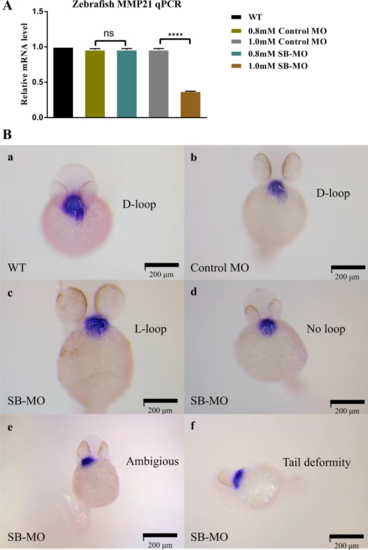
mmp21 knockdown induced by SB-MO caused heart looping disorder in zebrafish embryos. A Relative mmp21 mRNA expression in zebrafish embryos injected with SB-MO and standard control MO at concentrations of 0.8 mM and 1.0 mM for 30 h was assessed by RT-qPCR. RT-qPCR results showed that microinjection of SB-MO (1.0 mM) significantly decreased the mmp21 mRNA expression in zebrafish embryos compared to the control MO group (data are shown as the mean ± SEM, one-way ANOVA with Tukey’s multiple comparisons test was used for statistical calculation, n = 3 independent experiments, ns: nonsignificant, ****p < 0.0001). B Whole-mount in situ hybridization using a cmlc2 antisense probe to detect cyclization of 48 hfp embryonic cardiac tubes after MO injection. a, b The WT and control MO groups showed normal heart looping (D-loop). c, d, e Zebrafish embryos in the SB-MO group formed an abnormal L-loop on the left side or manifested as ambiguous looping or no loop. f Some zebrafish embryos in the SB-MO group showed tail deformity, which may be caused by side effects of SB-MO. Scale bar, 200 μm |
|
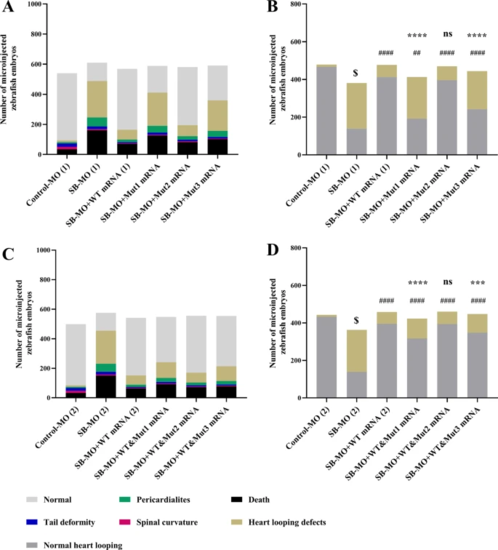
Microinjection of MMP21 mRNA c.731G > A and c.1459A > G failed to rescue the zebrafish heart looping disorder. The WT and mutant human MMP21 mRNAs synthesized using in vitro transcription were separately microinjected or co-microinjected (WT/mutant = 1:1) into 1–2 cell stage zebrafish embryos accompanied by SB-MO to compensate for the mmp21 knockdown. The zebrafish heart looping disorder phenotype at 48 hfp was observed and analyzed. Heart tube looping abnormalities include L-loop, ambiguous looping, or no looping. Left panels show all phenotypes after separate microinjection or co-microinjection of WT and mutant MMP21 mRNAs to zebrafish embryos. The right panels show normal and abnormal heart looping phenotypes. A, B Compared to the SB-MO group, MMP21 WT mRNA effectively reduced the number of malformed phenotypes. Mut1 and Mut3 could not effectively compensate for the effects of mmp21 knockdown. The effect of Mut2 was similar to that of the WT mRNA. C, D When co-microinjected with the WT and mutant mRNAs at a 1:1 ratio, no statistical difference in the malformation rate between the WT&Mut2 group and the WT mRNA group was noted. The malformation rate of the WT&Mu1 and WT&Mu3 groups was significantly reduced compared to that of the SB-MO group; however, it remained significantly higher than that of the WT mRNA group (chi-square test was used for statistical calculation, $ p < 0.0001 versus control MO group, #### p < 0.0001 versus SB-MO group, ## p < 0.01 versus SB-MO group, **** p < 0.0001 versus WT mRNA group, *** p < 0.001 versus WT mRNA group, ns: nonsignificant) PHENOTYPE:
|