- Title
-
Mice Lacking the Systemic Vitamin A Receptor RBPR2 Show Decreased Ocular Retinoids and Loss of Visual Function
- Authors
- Radhakrishnan, R., Leung, M., Roehrich, H., Walterhouse, S., Kondkar, A.A., Fitzgibbon, W., Biswal, M.R., Lobo, G.P.
- Source
- Full text @ Nutrients
|
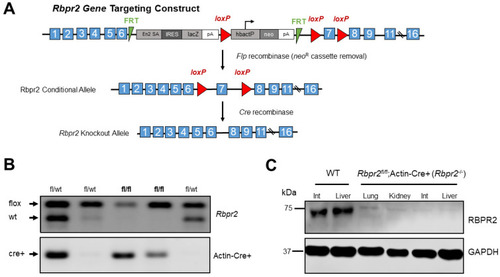
Disruption of the mouse Rbpr2 gene using the Cre/LoxP strategy and generation of the global Rbpr2 knockout (Rbpr2−/−) mice. (A) An Rbpr2 targeting vector was designed to replace exon 7 with a neomycin cassette (neoR) flanked by loxP sites. This resulted in the Rbpr2 neoR cassette allele. The neoR cassette was floxed out by mating Rbpr2fl/wt mice with the Rosaflp/flp mice. The Rbpr2fl/fl mice were then mated with Actin-Cre+ mice to finally generate littermate controls and the global Rbpr2 knockout (annotated as Rbpr2−/−) mice. (B) PCR-based genotyping for identifying the Rbpr2fl/fl; Actin-Cre+ (Rbpr2−/−) and littermate control mice. (C) A custom made RBPR2 antibody was generated and used to determine the systemic loss of RBPR2 protein expression in Rbpr2−/− mice. Immunoblot of protein extracts of tissues from adult WT and Rbpr2−/− mice. Pooled protein from n = 3 mice (per genotype) at 6–8 weeks of age. GAPDH antibody was used as the loading control. Int, intestine; WT, wild-type. |
|
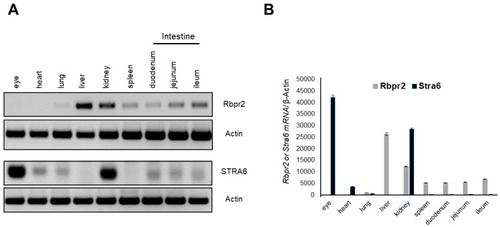
Rbpr2 and Stra6 mRNA expression in tissues of adult mice. (A) Tissues were harvested from adult wild-type (WT) mice (n = 3). Total RNA was isolated and pooled together, respectively. Semi-quantitative RT-PCR analysis for Rbpr2 and Stra6 mRNA expression was performed using β-actin as the loading control and the respective gene-specific primers. (B) Numbers on the ordinate represent Rbpr2 and Stra6 mRNA expression levels normalized to mouse β-actin and expressed as mean ± SEM. |
|
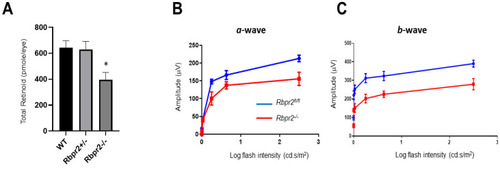
Rbpr2−/− mice on vitamin A-sufficient diets show decreased ocular retinoid concentration and loss of visual function. (A,B) Quantification of total retinoid concentrations (pmole/eye) in eyes from WT, Rbpr2+/−, and Rbpr2−/− mice (pooled eyes from n = 8 mice per genotype). * p < 0.05, Rbpr2−/− compared to controls. (C) Scotopic electroretinograms of 12-week old control (Rbpr2fl/fl) and Rbpr2−/− mice on vitamin A-sufficient (VAS) diets. * p < 0.05, for both a- and b-wave; Rbpr2−/− mice vs. controls. |
|
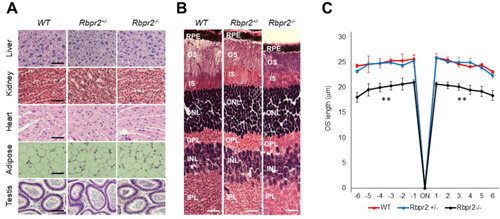
Rbpr2−/− mice on vitamin A-deficient diets show retinal phenotypes. (A,B) Representative tissue histology and staining of liver, heart, adipose, testis, retina (H&E stain), and kidney of 12-week old wild-type (WT), heterozygous Rbpr2+/−, and homozygous Rbpr2−/− (KO) mice on vitamin A-deficient diets. (C) Quantification of photoreceptor outer segment (OS) lengths from H&E sections of 12-week old WT, heterozygous Rbpr2+/−, and homozygous Rbpr2−/− (KO) mice using “spider graph” morphometry. The OS lengths from H&E sections through the optic nerve (ON; 0 μm distance from the optic nerve and starting point) were measured at 12 locations around the retina, six each in the superior and inferior hemispheres, each equally at approximately 150 μm distances. RPE, retinal pigmented epithelium; OS, outer segments; IS, inner segments; ONL, outer nuclear layer; INL, inner nuclear layer; IPL, inner plexiform layer. Scale bar = 50 µm (A); 100 µm (B). ** p < 0.005; Rbpr2−/− mice vs. controls. |
|
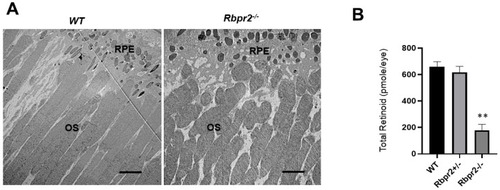
Rbpr2−/− mice on vitamin A-deficient diets show decreased ocular retinoid concentrations and shorter photoreceptor outer segments. (A) Ultrastructural analysis of photoreceptors using transmission electron microscopy (TEM): Representative TEM images of photoreceptors from 12-week old WT and Rbpr2−/− mice on vitamin A-deficient diets are presented. Scale bar = 600 μm. RPE, retinal pigmented epithelium; OS, outer segments. Data are representative of n = 6 retinal sections per eye from n = 4 mice per genotype. (B) Quantification of total retinoid concentrations (pmole/eye) in eyes from WT, Rbpr2+/−, and Rbpr2−/− mice (eyes from n = 8 mice per genotype). ** p < 0.005, Rbpr2−/− compared to controls. |
|
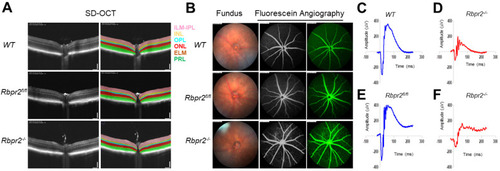
Rbpr2 is critical for photoreceptor cell viability and visual function under dietary vitamin A restriction. (A) OCT retinal analysis of 12-week old controls (WT, Rbpr2fl/fl) and homozygous Rbpr2−/− mice on vitamin A-deficient diets. Note that the photoreceptor cell layer is reduced (p < 0.05) in Rbpr2−/− mice compared to controls. (B) Fundus imaging of 12-week old wild-type (WT), floxed (Rbpr2fl/fl), and homozygous (Rbpr2−/−) mice after intraperitoneal injection with ICG (15 mg/kg, Acros Organics, NJ, USA). Note that blood vessel leakage was observed in Rbpr2−/− mice compared to controls. (C–F) Scotopic electroretinograms of 12-week old control (Rbpr2fl/fl) and Rbpr2−/− mice on vitamin A-deficient diets, Rbpr2−/− vs. controls at 0.1 cds/m2. |
|
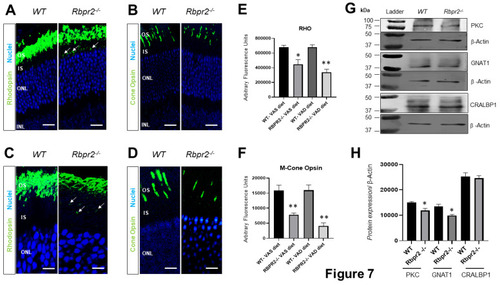
Immunohistochemical analysis for rhodopsin and cone opsin in retinas ofRbpr2−/−mice. Levels and localization of rhodopsin (Rho) in wild-type (WT) and Rbpr2−/− mice on either vitamin A-sufficient diets (A) or vitamin A-deficient diets (C). Red/green medium wavelength cone opsin (M-opsin) staining in WT and Rbpr2−/− mice on either vitamin A-sufficient diets (B) or vitamin A-deficient diets (D). Loss of cone opsin staining was observed in Rbpr2−/− mice under both dietary conditions (** p < 0.005). Rbpr2−/− mice on either vitamin A diet showed a significant loss of rhodopsin staining (* p < 0.05; ** p < 0.005) and mislocalization to the inner segments (arrows in panels A and C). Images in panels (A–D) are representative of immunostained retinal sections (n = 5–7 sections per eye) imaged from n = 8 animals per genotype and age group (50:50 male to female ratio). (E,F) Quantification of rhodopsin and cone-opsin in OS among various genotypes and dietary conditions (* p < 0.05; ** p < 0.005). Scale bars = 75 µm (A,B). Scale bars = 25 µm (C,D). OS, outer segments; IS, inner segments; ONL, outer nuclear layer; INL, inner nuclear layer. (G) Protein expression analysis of components of the photoreceptors. Western blot analysis for PKC, GNAT1, and CRALBP1 in total protein preparations of the retina in vitamin A-deficient Rbpr2−/− mice and WT mice (pooled n = 6 retinas per genotype and age). β-actin gene expression was used as the internal control. (H) Protein densitometry analysis and quantification. The statistical analysis was performed using an unpaired two-tail Student t-test by comparing values from age-matched Rbpr2−/− and WT mice. * p < 0.05. |
|
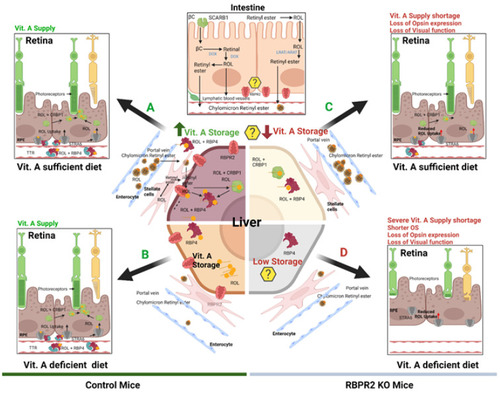
Schematic representation of the loss of vitamin A transport and its consequences on retinal homeostasis in Rbpr2−/− mice. Membrane receptor proteins mediate and recognize functions of RBP4 for ROL uptake. “Stimulated by retinoic acid 6” (STRA6) in the eye and “retinol binding protein receptor 2” (RBPR2, also known as STRA6Like) in liver and intestine are involved in retinol uptake and its coupling to RAR/RXR signaling in target cells. The specific RBP4 receptor in the liver (RBPR2) is thought to also mediate reverse transport of retinol (? Symbols), allowing a cycle of retinol between the circulation and liver. When compared to WT mice, Rbpr2−/− mice on either vitamin A-sufficient (VAS) or vitamin A-depleted diets (VAD) show reduced ocular retinoid concentrations and loss of visual function. Additionally, Rbpr2−/− mice show severe retinal phenotypes when challenged with a VAD diet. Thus, the retinal function impairment could be caused by a deficiency in systemic retinol transport to the eye, which is likely dependent on the function of RBPR2. The model was created with BioRender.com (https://help.biorender.com/en/articles/3619405-how-do-i-cite-biorender, accessed on 5 January 2022). |