- Title
-
Bactericidal Synergism between Phage YC#06 and Antibiotics: a Combination Strategy to Target Multidrug-Resistant Acinetobacter baumannii In Vitro and In Vivo
- Authors
- Luo, J., Xie, L., Liu, M., Li, Q., Wang, P., Luo, C.
- Source
- Full text @ Microbiol Spectr
|
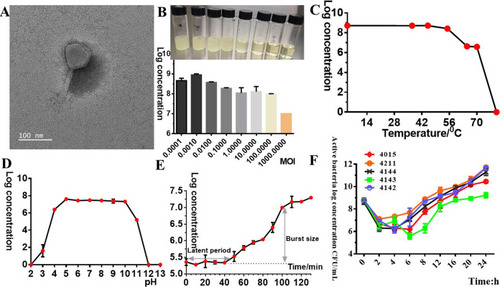
(A) Transmission electron micrograph of phage YC#06 virions negatively stained with 2% phosphotungstic acid. (B to E) Biological properties of phage YC#06. Determination of the optimal MOI (B), temperature tolerance (C), pH sensitivity (D), and one-step growth curve (E). (F) Dynamic curves of bacterial escape from phage control based on viable bacteria counting observation. Phage titers were measured by the double-layer agar method. Data are presented as the mean ± standard deviation (SD). |
|
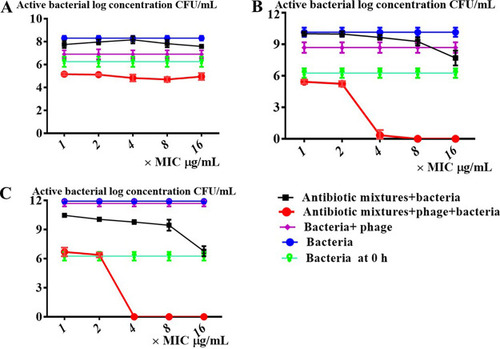
Effect of phage dose on PAS between YC#06 and antibiotic combination. The |
|
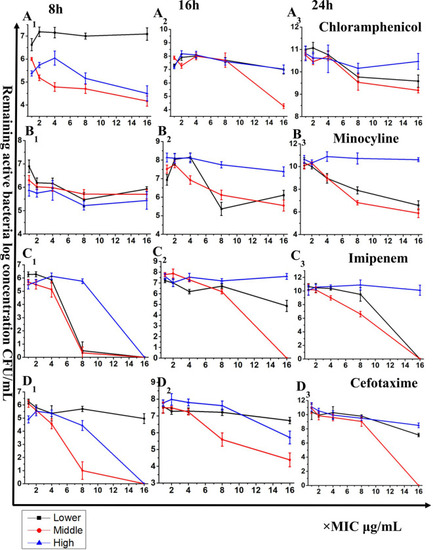
Time-kill analyses of antibiotic mixtures alone and in combination with bacteriophage YC#06 against |
|
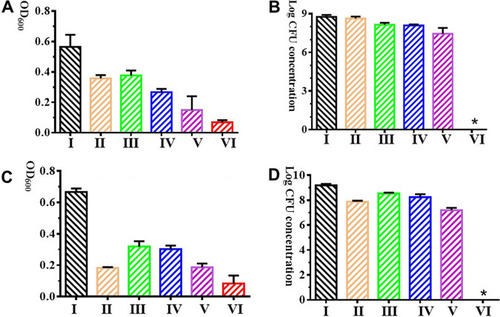
Antibiofilm effect of antibiotic mixtures alone and in combination with bacteriophage YC#06 on biofilm formation inhibition (A, B) and mature biofilm reduction (C, D) |
|
PHENOTYPE:
|