- Title
-
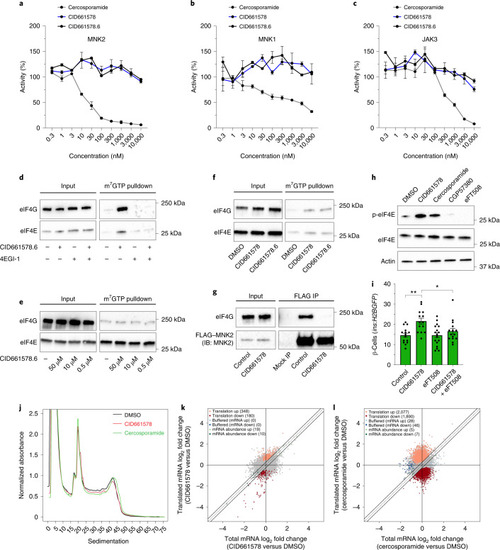
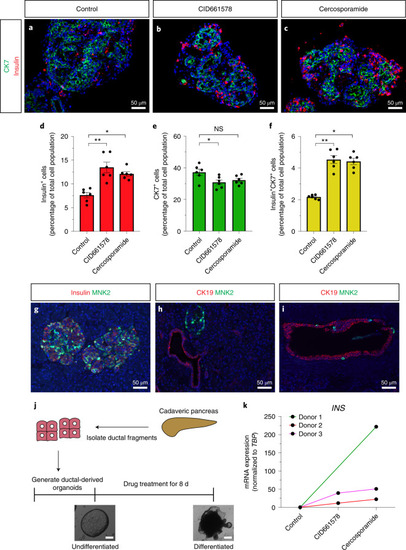
MNK2 deficiency potentiates β-cell regeneration via translational regulation
- Authors
- Karampelias, C., Watt, K., Mattsson, C.L., Ruiz, Á.F., Rezanejad, H., Mi, J., Liu, X., Chu, L., Locasale, J.W., Korbutt, G.S., Rovira, M., Larsson, O., Andersson, O.
- Source
- Full text @ Nat. Chem. Biol.
|
|
|
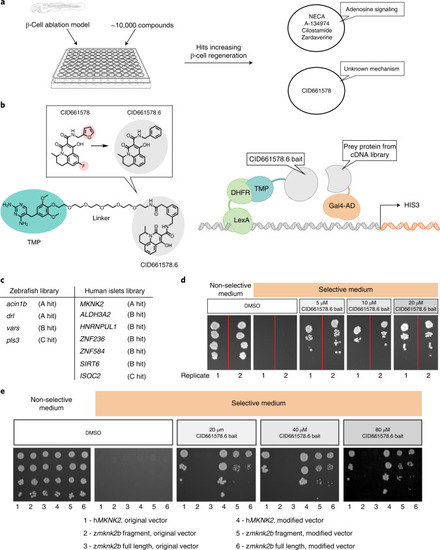
EXPRESSION / LABELING:
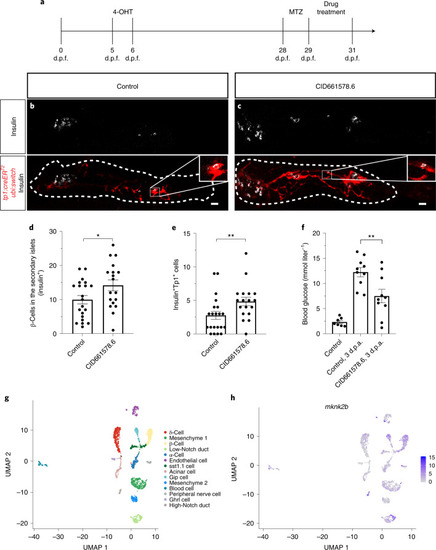
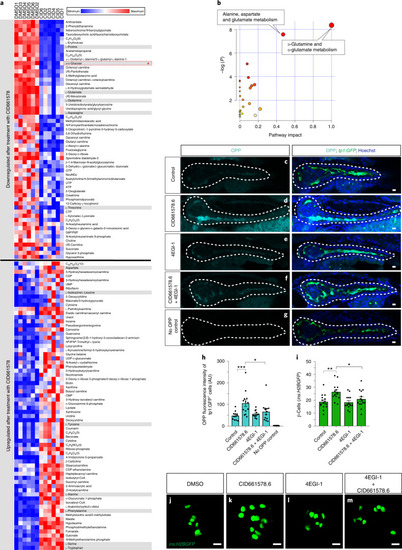
PHENOTYPE:
|
|
|
|
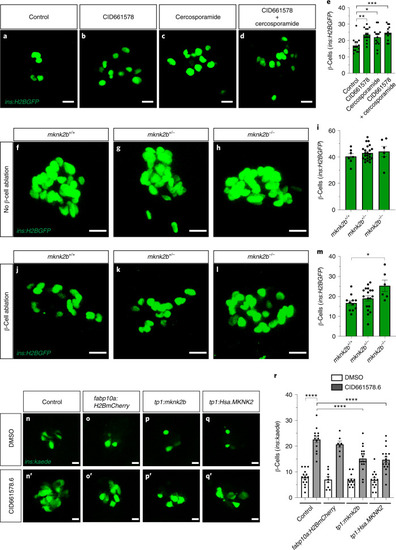
PHENOTYPE:
|
|
|
|
|